Employing fasting plasma glucose to safely limit the use of oral glucose tolerance tests in pregnancy: a pooled analysis of four Norwegian studies
- PMID: 38098869
- PMCID: PMC10720624
- DOI: 10.3389/fendo.2023.1278523
Employing fasting plasma glucose to safely limit the use of oral glucose tolerance tests in pregnancy: a pooled analysis of four Norwegian studies
Abstract
Background/objective: There is no international consensus about the optimal approach to screening and diagnosis of gestational diabetes mellitus (GDM). Fasting plasma glucose (FPG) has been proposed as an alternative universal screening test to simplify the diagnosis of GDM. We investigate the ability of the FPG to predict a 2-hour glucose value below the cut-off for GDM, thereby "ruling out" the necessity of a full OGTT and assess the proportion of GDM-related complications associated with the identified FPG level.
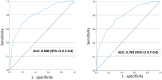
Materials and methods: This study included secondary data from four Norwegian pregnancy cohorts (2002-2013), encompassing 2960 women universally screened with late mid-pregnancy 75g OGTT measuring FPG and 2-hour glucose. For a range of FPG thresholds, we calculated sensitivity to predict elevated 2-hour glucose, number of OGTTs needed and percentage of GDM cases missed, applying modified World Health Organization (WHO) 2013 criteria (2013WHO) and 2017 Norwegian criteria (2017Norwegian). We analyzed pregnancy outcomes for women above and below our selected threshold.
Results: The prevalence of GDM was 16.6% (2013WHO) and 10.1% (2017Norwegian). A FPG threshold of 4.7 mmol/L had a sensitivity of 76% (2013WHO) and 80% (2017Norwegian) for detecting elevated 2-hour glucose, with few missed GDM cases (2.0% of those ruled out and 7.5% of all GDM cases for 2013WHO, and 1.1% of those ruled out and 7% of all GDM cases for 2017Norwegian). When excluding women with FPG <4.7mmol/l and those with GDM based on FPG, only 24% (2013WHO) and 29% (2017Norwegian) would require OGTT. Women with FPG <4.7mmol/l, including missed GDM cases, had low risk of large-for-gestational-age newborns, cesarean section and operative vaginal delivery.
Conclusion: A FPG threshold of 4.7mmol/l as a first step when screening for GDM could potentially eliminate the need for OGTT in 70-77% of pregnancies. Women with FPG below this threshold appear to carry low risk of GDM-associated adverse pregnancy outcomes.
Keywords: OGTT 3; fasting plasma glucose; gestational diabetes; pregnancy outcomes; screening.
Copyright © 2023 Rai, Sletner, Jenum, Øverby, Stafne, Qvigstad, Pripp and Sagedal.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Hod M, Kapur A, Sacks DA, Hadar E, Agarwal M, Di Renzo GC, et al. The International Federation of Gynecology and Obstetrics (FIGO) Initiative on gestational diabetes mellitus: A pragmatic guide for diagnosis, management, and care. Int J Gynaecol Obstet. (2015) 131(Suppl 3):S173–211. doi: 10.1016/S0020-7292(15)30007-2 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical