Spinal cord stimulation in severe pharmacoresistant restless legs syndrome-two case reports
- PMID: 38099065
- PMCID: PMC10720037
- DOI: 10.3389/fneur.2023.1219881
Spinal cord stimulation in severe pharmacoresistant restless legs syndrome-two case reports
Abstract
Restless legs syndrome is a prevalent, sleep-related sensorimotor disorder with relevant impact on the patients' quality of life. For patients suffering from severe, pharmacoresistant restless legs syndrome, few therapeutic options remain to alleviate symptoms. In this case series, two patients with severe, pharmacoresistant restless legs syndrome were treated with epidural spinal cord stimulation and repeatedly assessed with polysomnography, including sleep structure and periodic limb movements as objective biomarkers not subject to placebo effects, during a 6-month follow-up period. One of the patients experienced excellent short- and long-term efficacy on subjective symptom severity (International RLS Study group rating scale 1 vs. 34 points at 3 months) and objective sleep parameters such as sleep architecture and periodic limb movements during sleep, while the second patient only reported short-term benefits from spinal cord stimulation. Ultimately, both patients opted for removal of the device for inefficacy. Based on the complex pathophysiology of restless legs syndrome and presumed mechanism of action of spinal cord stimulation in chronic pain disorders, we provide a detailed hypothesis on the possible modulating effect of spinal cord stimulation on the key symptoms of restless legs syndrome. Apart from describing a new therapeutic option for pharmacoresistant restless legs syndrome, our findings might also provide further insights into the pathophysiology of the syndrome.
Keywords: augmentation; case report; epidural spinal cord stimulation; periodic limb movements during sleep; restless legs syndrome.
Copyright © 2023 Hackethal, Maino, Koetsier and Manconi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
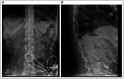
Figures



References
-
- Allen RP, Picchietti DL, Garcia-Borreguero D, Ondo WG, Walters AS, Winkelman JW, et al. . Lee HB; international restless legs syndrome study group. Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: updated international restless legs syndrome study group (IRLSSG) consensus criteria--history, rationale, description, and significance. Sleep Med. (2014) 15:860–73. doi: 10.1016/j.sleep.2014.03.025, PMID: - DOI - PubMed
-
- García-Borreguero D, Allen RP, Kohnen R, Högl B, Trenkwalder C, Oertel W, et al. . Earley CJ; international restless legs syndrome study group. Diagnostic standards for dopaminergic augmentation of restless legs syndrome: report from a World Association of Sleep Medicine-International Restless Legs Syndrome Study Group consensus conference at the max Planck institute. Sleep Med. (2007) 8:520–30. doi: 10.1016/j.sleep.2007.03.022, PMID: - DOI - PubMed
-
- Garcia-Borreguero D, Silber MH, Winkelman JW, Högl B, Bainbridge J, Buchfuhrer M, et al. . Guidelines for the first-line treatment of restless legs syndrome/Willis-Ekbom disease, prevention and treatment of dopaminergic augmentation: a combined task force of the IRLSSG, EURLSSG, and the RLS-foundation. Sleep Med. (2016) 21:1–11. doi: 10.1016/j.sleep.2016.01.017, PMID: - DOI - PubMed

