Harnessing the translational power of bleomycin model: new insights to guide drug discovery for idiopathic pulmonary fibrosis
- PMID: 38099140
- PMCID: PMC10719847
- DOI: 10.3389/fphar.2023.1303646
Harnessing the translational power of bleomycin model: new insights to guide drug discovery for idiopathic pulmonary fibrosis
Abstract
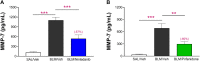
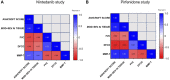
Background: Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive, age-related interstitial lung disease (ILD) with limited therapeutic options. Despite the wide variety of different in vivo models for IPF, these preclinical models have shown limitations that may significantly impair their translational potential. Among the most relevant limitations are the methodologies used to assess the efficacy of anti-fibrotic treatments, that are not the ones used in humans. In this scenario, the goal of the work presented in this paper is to provide translational relevance to the bleomycin (BLM)-induced pulmonary fibrosis mouse model, introducing and validating novel readouts to evaluate the efficacy of treatments for IPF. Methods: The BLM model was optimized by introducing the use of functional assessments such as the Forced Vital Capacity (FVC) and the Diffusion Factor for Carbon Monoxide (DFCO), that are respectively the primary and secondary endpoints in clinical trials for IPF, comparing them to more common readouts such as lung histology, improved by the application of Artificial Intelligence (AI) to detect and quantify fibrotic tissue deposition, and metalloproitenase-7 (MMP-7), a clinical prognostic biomarker. Results: Lung function measurement and DFCO changes well correlated with Ashcroft score, the current gold-standard for the assessment of pulmonary fibrosis in mice. The relevance and robustness of these novel readouts in the BLM model was confirmed by the results obtained testing Nintedanib and Pirfenidone, the only drugs approved for the treatment of IPF patients: in fact, both drugs administered therapeutically, significantly affected the changes in these parameters induced by BLM treatment, with results that closely reflected the efficacy observed in the clinic. Changes in biomarkers such as MMP-7 were also evaluated, and well correlated with the modifications of FVC and DFCO. Conclusion: Novel functional readouts such as FVC and DFCO can be efficiently used to assess pathology progression in the BLM-induced pulmonary fibrosis mouse model as well as compound efficacy, substantially improving its translational and predictivity potential.
Keywords: Ashcroft score; Broncho alveolar lavage fluid; Nintedanib; Pirfenidone; automated histology imaging analysis; diffusion factor for carbon monoxide; forced vital capacity; metalloproteinase-7.
Copyright © 2023 Murgo, Bignami, Federico, Villetti, Civelli, Sala and Miglietta.
Conflict of interest statement
Authors AM, FB, GF, GV, MC and DM were employed by the company Chiesi Farmaceutici S.p.A. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures








References
-
- Durheim M. T., Collard H. R., Roberts R. S., Brown K. K., Flaherty K. R., King T. E., et al. (2015). Association of hospital admission and forced vital capacity endpoints with survival in patients with idiopathic pulmonary fibrosis: analysis of a pooled cohort from three clinical trials. Lancet Respir. Med. 3, 388–396. 10.1016/S2213-2600(15)00093-4 - DOI - PMC - PubMed

