HEG1 Protects Against Atherosclerosis by Regulating Stable Flow-Induced KLF2/4 Expression in Endothelial Cells
- PMID: 38099436
- PMCID: PMC11001532
- DOI: 10.1161/CIRCULATIONAHA.123.064735
HEG1 Protects Against Atherosclerosis by Regulating Stable Flow-Induced KLF2/4 Expression in Endothelial Cells
Abstract
Background: Atherosclerosis preferentially occurs in arterial regions of disturbed blood flow, and stable flow (s-flow) protects against atherosclerosis by incompletely understood mechanisms.
Methods: Our single-cell RNA-sequencing data using the mouse partial carotid ligation model was reanalyzed, which identified Heart-of-glass 1 (HEG1) as an s-flow-induced gene. HEG1 expression was studied by immunostaining, quantitive polymerase chain reaction, hybridization chain reaction, and Western blot in mouse arteries, human aortic endothelial cells (HAECs), and human coronary arteries. A small interfering RNA-mediated knockdown of HEG1 was used to study its function and signaling mechanisms in HAECs under various flow conditions using a cone-and-plate shear device. We generated endothelial-targeted, tamoxifen-inducible HEG1 knockout (HEG1iECKO) mice. To determine the role of HEG1 in atherosclerosis, HEG1iECKO and littermate-control mice were injected with an adeno-associated virus-PCSK9 [proprotein convertase subtilisin/kexin type 9] and fed a Western diet to induce hypercholesterolemia either for 2 weeks with partial carotid ligation or 2 months without the surgery.
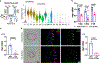
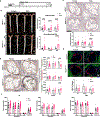
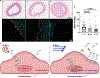
Results: S-flow induced HEG1 expression at the mRNA and protein levels in vivo and in vitro. S-flow stimulated HEG1 protein translocation to the downstream side of HAECs and release into the media, followed by increased messenger RNA and protein expression. HEG1 knockdown prevented s-flow-induced endothelial responses, including monocyte adhesion, permeability, and migration. Mechanistically, HEG1 knockdown prevented s-flow-induced KLF2/4 (Kruppel-like factor 2/4) expression by regulating its intracellular binding partner KRIT1 (Krev interaction trapped protein 1) and the MEKK3-MEK5-ERK5-MEF2 pathway in HAECs. Compared with littermate controls, HEG1iECKO mice exposed to hypercholesterolemia for 2 weeks and partial carotid ligation developed advanced atherosclerotic plaques, featuring increased necrotic core area, thin-capped fibroatheroma, inflammation, and intraplaque hemorrhage. In a conventional Western diet model for 2 months, HEG1iECKO mice also showed an exacerbated atherosclerosis development in the arterial tree in both sexes and the aortic sinus in males but not in females. Moreover, endothelial HEG1 expression was reduced in human coronary arteries with advanced atherosclerotic plaques.
Conclusions: Our findings indicate that HEG1 is a novel mediator of atheroprotective endothelial responses to flow and a potential therapeutic target.
Keywords: KLF2/4; atherosclerosis; endothelial cells; heart-of-glass 1; mechanobiology.
Conflict of interest statement
Figures





Comment in
-
Hemodynamic Forces and Atherosclerosis: HEG1 at the Center of the Jigsaw Puzzle.Circulation. 2024 Apr 9;149(15):1202-1204. doi: 10.1161/CIRCULATIONAHA.124.067882. Epub 2024 Apr 8. Circulation. 2024. PMID: 38588335 Free PMC article. No abstract available.
References
-
- Caro CG, Fitz-Gerald JM and Schroter RC. Arterial wall shear and distribution of early atheroma in man. Nature. 1969;223:1159–60. - PubMed
-
- Malek AM, Alper SL and Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA. 1999;282:2035–42. - PubMed
-
- VanderLaan PA, Reardon CA and Getz GS. Site specificity of atherosclerosis: site-selective responses to atherosclerotic modulators. Arterioscler Thromb Vasc Biol. 2004;24:12–22. - PubMed
-
- Li YS, Haga JH and Chien S. Molecular basis of the effects of shear stress on vascular endothelial cells. J Biomech. 2005;38:1949–71. - PubMed
-
- Demos C, Tamargo I and Jo H. Biomechanical regulation of endothelial function in atherosclerosis. In: Ohayon J, Finet G and Pettigrew R, eds. Biomechanics of Coronary Atherosclerotic Plaque: Academic Press; 2021(4): 3–47.
Publication types
MeSH terms
Substances
Grants and funding
- F31 HL145974/HL/NHLBI NIH HHS/United States
- T32 GM142617/GM/NIGMS NIH HHS/United States
- R01 HL139757/HL/NHLBI NIH HHS/United States
- T32 HL007745/HL/NHLBI NIH HHS/United States
- R01 HL168383/HL/NHLBI NIH HHS/United States
- R01 HL119798/HL/NHLBI NIH HHS/United States
- F31 HL149285/HL/NHLBI NIH HHS/United States
- F32 HL167625/HL/NHLBI NIH HHS/United States
- T32 HL166146/HL/NHLBI NIH HHS/United States
- R01 HL158571/HL/NHLBI NIH HHS/United States
- T32 GM008433/GM/NIGMS NIH HHS/United States
- T32 GM008602/GM/NIGMS NIH HHS/United States
- HL151358/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

