Remodelling of cAMP dynamics within the SERCA2a microdomain in heart failure with preserved ejection fraction caused by obesity and type 2 diabetes
- PMID: 38099489
- PMCID: PMC10939460
- DOI: 10.1093/cvr/cvad178
Remodelling of cAMP dynamics within the SERCA2a microdomain in heart failure with preserved ejection fraction caused by obesity and type 2 diabetes
Abstract
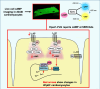
Aims: Despite massive efforts, we remain far behind in our attempts to identify effective therapies to treat heart failure with preserved ejection fraction (HFpEF). Diastolic function is critically regulated by sarcoplasmic/endoplasmic reticulum (SR) calcium ATPase 2a (SERCA2a), which forms a functional cardiomyocyte (CM) microdomain where 3',5'-cyclic adenosine monophosphate (cAMP) produced upon β-adrenergic receptor (β-AR) stimulation leads to phospholamban (PLN) phosphorylation and facilitated Ca2+ re-uptake.
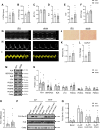
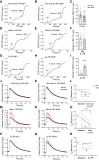
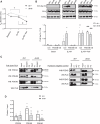
Methods and results: To visualize real-time cAMP dynamics in the direct vicinity of SERCA2a in healthy and diseased myocytes, we generated a novel mouse model on the leprdb background that stably expresses the Epac1-PLN Förster resonance energy transfer biosensor. Mice homozygous for the leprdb mutation (db/db) developed obesity and type 2 diabetes and presented with a HFpEF phenotype, evident by mild left ventricular hypertrophy and elevated left atria filling pressures. Live cell imaging uncovered a substantial β2-AR subtype stimulated cAMP response within the PLN/SERCA2a microdomain of db/db but not healthy control (db/+) CMs, which was accompanied by increased PLN phosphorylation and accelerated calcium re-uptake. Importantly, db/db CMs also exhibited a desensitization of β1-AR stimulated cAMP pools within the PLN/SERCA2a microdomain, which was accompanied by a blunted lusitropic effect, suggesting that the increased β2-AR control is an intrinsic compensatory mechanism to maintain PLN/SERCA2a-mediated calcium dynamics and cardiac relaxation. Mechanistically, this was due to a local loss of cAMP-degrading phosphodiesterase 4 associated specifically with the PLN/SERCA2a complex.
Conclusion: These newly identified alterations of cAMP dynamics at the subcellular level in HFpEF should provide mechanistic understanding of microdomain remodelling and pave the way towards new therapies.
Keywords: 3′, 5′-Cyclic adenosine monophosphate; Förster resonance energy transfer; Heart failure with preserved ejection fraction; Obesity; Phosphodiesterase; Phospholamban; Sarcoplasmic/endoplasmic reticulum calcium ATPase 2a; Type 2 diabetes.
© The Author(s) 2023. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: none declared.
Figures

Comment in
-
SERCA2a microdomain cAMP changes in heart failure with preserved ejection fraction.Cardiovasc Res. 2024 Mar 14;120(3):220-222. doi: 10.1093/cvr/cvae030. Cardiovasc Res. 2024. PMID: 38333928 No abstract available.
References
-
- Savji N, Meijers WC, Bartz TM, Bhambhani V, Cushman M, Nayor M, Kizer JR, Sarma A, Blaha MJ, Gansevoort RT, Gardin JM, Hillege HL, Ji F, Kop WJ, Lau ES, Lee DS, Sadreyev R, van Gilst WH, Wang TJ, Zanni MV, Vasan RS, Allen NB, Psaty BM, van der Harst P, Levy D, Larson M, Shah SJ, de Boer RA, Gottdiener JS, Ho JE. The association of obesity and cardiometabolic traits with incident HFpEF and HFrEF. JACC Heart Fail 2018;6:701–709. - PMC - PubMed
-
- MacDonald MR, Petrie MC, Varyani F, Ostergren J, Michelson EL, Young JB, Solomon SD, Granger CB, Swedberg K, Yusuf S, Pfeffer MA, McMurray JJ, Investigators C. Impact of diabetes on outcomes in patients with low and preserved ejection fraction heart failure: an analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) programme. Eur Heart J 2008;29:1377–1385. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

