Real-world use of inotuzumab ozogamicin is associated with lower health care costs than blinatumomab in patients with acute lymphoblastic leukemia in the first relapsed/refractory setting
- PMID: 38099517
- PMCID: PMC10842295
- DOI: 10.57264/cer-2023-0142
Real-world use of inotuzumab ozogamicin is associated with lower health care costs than blinatumomab in patients with acute lymphoblastic leukemia in the first relapsed/refractory setting
Abstract
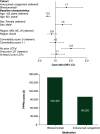
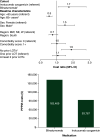
Aim: To compare all-cause and acute lymphoblastic leukemia (ALL)-related healthcare resource utilization (HCRU) and costs among patients receiving inotuzumab ozogamicin (InO) and blinatumomab (Blina) for ALL in the first relapsed/refractory (R/R) setting. Patients & methods: We studied retrospective claims for adult commercial and Medicare Advantage enrollees with ALL receiving InO (n = 29) or Blina (n = 23) from 1 January 2015 to 16 February 2021. Mean per-patient-per-month (PPPM) HCRU and total costs were described and multivariable-adjusted PPPM total all-cause and ALL-related predicted costs were calculated. Results: Mean monthly ALL-related hospitalizations were the same for patients receiving InO and Blina (PPPM = 0.8 stays); however, the length of ALL-related hospital stay was almost twice as long among patients receiving Blina versus InO (ALL-related: InO = 7.6 days; Blina = 14.1 days; p = 0.346). In multivariable models, total ALL-related costs were 43% lower for InO compared with Blina (PPPM costs: InO = $93,767; Blina = $163,470; p = 0.021). Conclusion: In the first R/R setting, patients who used InO had significantly lower all-cause and ALL-related costs compared with patients who used Blina, in part driven by hospitalization patterns.
Keywords: acute lymphoblastic leukemia; costs; health care resource utilization; medications; relapse/refractory.
Conflict of interest statement
Competing interests disclosure
A Russell-Smith, S Dorman and R Shah are employees of, and own stock in, Pfizer. L Murphy and T Bancroft are employees of Optum and own stock in UnitedHealth Group. A Nguyen, C Blauer-Peterson, F Cao, S Li and N Webb are employees of Optum. M Terpenning has received funds for consulting with Optum and Curio Sciences. The authors have no other competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript apart from those disclosed.
Figures
References
-
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J. Clin. 72(1), 7–33 (2022). - PubMed
-
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Acute Lymphoblastic Leukemia. Version 2.2022. (2023). www.nccn.org/professionals/physician_gls/default.aspx#all
-
- Gorin NC. Autologous stem cell transplantation in acute lymphocytic leukemia. Stem Cells 20(1), 3–10 (2022). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
