Implications for neutrophils in cardiac arrhythmias
- PMID: 38099844
- PMCID: PMC11219058
- DOI: 10.1152/ajpheart.00590.2023
Implications for neutrophils in cardiac arrhythmias
Abstract
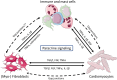
Cardiac arrhythmias commonly occur as a result of aberrant electrical impulse formation or conduction in the myocardium. Frequently discussed triggers include underlying heart diseases such as myocardial ischemia, electrolyte imbalances, or genetic anomalies of ion channels involved in the tightly regulated cardiac action potential. Recently, the role of innate immune cells in the onset of arrhythmic events has been highlighted in numerous studies, correlating leukocyte expansion in the myocardium to increased arrhythmic burden. Here, we aim to call attention to the role of neutrophils in the pathogenesis of cardiac arrhythmias and their expansion during myocardial ischemia and infectious disease manifestation. In addition, we will elucidate molecular mechanisms associated with neutrophil activation and discuss their involvement as direct mediators of arrhythmogenicity.
Keywords: arrhythmia; immune system; mechanisms; molecular; neutrophils.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


References
-
- Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 117: e25–e146, 2008. [Erratum in Circulation 122: e10, 2010]. doi:10.1161/CIRCULATIONAHA.107.187998. - DOI - PubMed
-
- Rozen G, Hosseini SM, Kaadan MI, Biton Y, Heist EK, Vangel M, Mansour MC, Ruskin JN. Emergency department visits for atrial fibrillation in the United States: trends in admission rates and economic burden from 2007 to 2014. J Am Heart Assoc 7: e009024, 2018. doi:10.1161/JAHA.118.009024. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
- Deutsches Zentrum für Herz-Kreislaufforschung (DZHK)
- CRC-1470 A04/Deutsche Forschungsgemeinschaft (DFG)
- DynAge/Freie Universität Berlin (FU)
- Deutsche Gesellschaft für Kardiologie-Herz und Kreislaufforschung. (DGK)
- Corona-Stiftung (Corona Foundation)
- Deutsche Stiftung für Herzforschung (German Heart Research Foundation)
- Charité 3R/Charité - Universitätsmedizin Berlin (Medical School - Charité - University Medicine Berlin)
- VO 1568/3-1, VO 1568/4-1, SFB1002 A13, EXC 2067/1-390729940/Deutsche Forschungsgemeinschaft (DFG)
- 81X4300120, 81X4300102/Deutsches Zentrum für Herz-Kreislaufforschung (DZHK)
LinkOut - more resources
Full Text Sources
Miscellaneous

