Genotyping of European Toxoplasma gondii strains by a new high-resolution next-generation sequencing-based method
- PMID: 38099986
- PMCID: PMC10822014
- DOI: 10.1007/s10096-023-04721-7
Genotyping of European Toxoplasma gondii strains by a new high-resolution next-generation sequencing-based method
Abstract
Purpose: A new high-resolution next-generation sequencing (NGS)-based method was established to type closely related European type II Toxoplasma gondii strains.
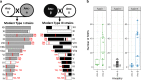
Methods: T. gondii field isolates were collected from different parts of Europe and assessed by whole genome sequencing (WGS). In comparison to ME49 (a type II reference strain), highly polymorphic regions (HPRs) were identified, showing a considerable number of single nucleotide polymorphisms (SNPs). After confirmation by Sanger sequencing, 18 HPRs were used to design a primer panel for multiplex PCR to establish a multilocus Ion AmpliSeq typing method. Toxoplasma gondii isolates and T. gondii present in clinical samples were typed with the new method. The sensitivity of the method was tested with serially diluted reference DNA samples.
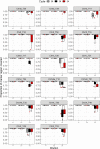
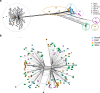
Results: Among type II specimens, the method could differentiate the same number of haplotypes as the reference standard, microsatellite (MS) typing. Passages of the same isolates and specimens originating from abortion outbreaks were identified as identical. In addition, seven different genotypes, two atypical and two recombinant specimens were clearly distinguished from each other by the method. Furthermore, almost all SNPs detected by the Ion AmpliSeq method corresponded to those expected based on WGS. By testing serially diluted DNA samples, the method exhibited a similar analytical sensitivity as MS typing.
Conclusion: The new method can distinguish different T. gondii genotypes and detect intra-genotype variability among European type II T. gondii strains. Furthermore, with WGS data additional target regions can be added to the method to potentially increase typing resolution.
Keywords: Discriminatory power; Highly polymorphic regions; Intra-genotype variability; Multilocus sequence typing; Toxoplasmosis; Typing.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Dubey JP. Toxoplasmosis of animals and humans. Third. Boca Raton: CRC Press; 2022.

