α-Gal Nanoparticles in CNS Trauma: I. In Vitro Activation of Microglia Towards a Pro-Healing State
- PMID: 38099990
- PMCID: PMC10987450
- DOI: 10.1007/s13770-023-00613-1
α-Gal Nanoparticles in CNS Trauma: I. In Vitro Activation of Microglia Towards a Pro-Healing State
Abstract
Background: Macrophages and microglia play critical roles after spinal cord injury (SCI), with the pro-healing, anti-inflammatory (M2) subtype being implicated in tissue repair. We hypothesize that promoting this phenotype within the post-injured cord microenvironment may provide beneficial effects for mitigating tissue damage. As a proof of concept, we propose the use of nanoparticles incorporating the carbohydrate antigen, galactose-α-1,3-galactose (α-gal epitope) as an immunomodulator to transition human microglia (HMC3) cells toward a pro-healing state.
Methods: Quiescent HMC3 cells were acutely exposed to α-gal nanoparticles in the presence of human serum and subsequently characterized for changes in cell shape, expression of anti or pro-inflammatory markers, and secretion of phenotype-specific cytokines.
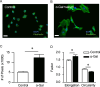
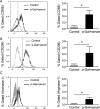
Results: HMC3 cells treated with serum activated α-gal nanoparticles exhibited rapid enlargement and shape change in addition to expressing CD68. Moreover, these activated cells showed increased expression of anti-inflammatory markers like Arginase-1 and CD206 without increasing production of pro-inflammatory cytokines TNF-α or IL-6.
Conclusion: This study is the first to show that resting human microglia exposed to a complex of α-gal nanoparticles and anti-Gal (from human serum) can be activated and polarized toward a putative M2 state. The data suggests that α-gal nanoparticles may have therapeutic relevance to the CNS microenvironment, in both recruiting and polarizing macrophages/microglia at the application site. The immunomodulatory activity of these α-gal nanoparticles post-SCI is further described in the companion work (Part II).
Keywords: Anti-inflammatory; Cytokines; Immunomodulation; Microglia; Spinal cord injury; α-gal.
© 2023. Korean Tissue Engineering and Regenerative Medicine Society.
Conflict of interest statement
The authors have not disclosed any conflict of interest.
Figures



Similar articles
-
α-Gal Nanoparticles in CNS Trauma: II. Immunomodulation Following Spinal Cord Injury (SCI) Improves Functional Outcomes.Tissue Eng Regen Med. 2024 Apr;21(3):437-453. doi: 10.1007/s13770-023-00616-y. Epub 2024 Feb 3. Tissue Eng Regen Med. 2024. PMID: 38308742 Free PMC article.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Single-cell RNA sequencing integrated with bulk RNA sequencing analysis reveals the protective effects of lactate-mediated lactylation of microglia-related proteins on spinal cord injury.CNS Neurosci Ther. 2024 Sep;30(9):e70028. doi: 10.1111/cns.70028. CNS Neurosci Ther. 2024. PMID: 39218784 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2020 Jan 9;1(1):CD011535. doi: 10.1002/14651858.CD011535.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 19;4:CD011535. doi: 10.1002/14651858.CD011535.pub4. PMID: 31917873 Free PMC article. Updated.
Cited by
-
Regeneration in Mice of Injured Skin, Heart, and Spinal Cord by α-Gal Nanoparticles Recapitulates Regeneration in Amphibians.Nanomaterials (Basel). 2024 Apr 22;14(8):730. doi: 10.3390/nano14080730. Nanomaterials (Basel). 2024. PMID: 38668224 Free PMC article. Review.
-
Modulation of Inflammatory Responses to Enhance Nerve Recovery after Spinal Cord Injury.Tissue Eng Regen Med. 2024 Apr;21(3):367-368. doi: 10.1007/s13770-024-00639-z. Epub 2024 Mar 26. Tissue Eng Regen Med. 2024. PMID: 38530570 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
