α-Gal Nanoparticles in CNS Trauma: I. In Vitro Activation of Microglia Towards a Pro-Healing State
- PMID: 38099990
- PMCID: PMC10987450
- DOI: 10.1007/s13770-023-00613-1
α-Gal Nanoparticles in CNS Trauma: I. In Vitro Activation of Microglia Towards a Pro-Healing State
Abstract
Background: Macrophages and microglia play critical roles after spinal cord injury (SCI), with the pro-healing, anti-inflammatory (M2) subtype being implicated in tissue repair. We hypothesize that promoting this phenotype within the post-injured cord microenvironment may provide beneficial effects for mitigating tissue damage. As a proof of concept, we propose the use of nanoparticles incorporating the carbohydrate antigen, galactose-α-1,3-galactose (α-gal epitope) as an immunomodulator to transition human microglia (HMC3) cells toward a pro-healing state.
Methods: Quiescent HMC3 cells were acutely exposed to α-gal nanoparticles in the presence of human serum and subsequently characterized for changes in cell shape, expression of anti or pro-inflammatory markers, and secretion of phenotype-specific cytokines.
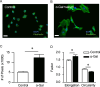
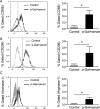
Results: HMC3 cells treated with serum activated α-gal nanoparticles exhibited rapid enlargement and shape change in addition to expressing CD68. Moreover, these activated cells showed increased expression of anti-inflammatory markers like Arginase-1 and CD206 without increasing production of pro-inflammatory cytokines TNF-α or IL-6.
Conclusion: This study is the first to show that resting human microglia exposed to a complex of α-gal nanoparticles and anti-Gal (from human serum) can be activated and polarized toward a putative M2 state. The data suggests that α-gal nanoparticles may have therapeutic relevance to the CNS microenvironment, in both recruiting and polarizing macrophages/microglia at the application site. The immunomodulatory activity of these α-gal nanoparticles post-SCI is further described in the companion work (Part II).
Keywords: Anti-inflammatory; Cytokines; Immunomodulation; Microglia; Spinal cord injury; α-gal.
© 2023. Korean Tissue Engineering and Regenerative Medicine Society.
Conflict of interest statement
The authors have not disclosed any conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
