OsGELP77, a QTL for broad-spectrum disease resistance and yield in rice, encodes a GDSL-type lipase
- PMID: 38100249
- PMCID: PMC11022805
- DOI: 10.1111/pbi.14271
OsGELP77, a QTL for broad-spectrum disease resistance and yield in rice, encodes a GDSL-type lipase
Abstract
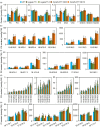
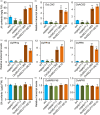
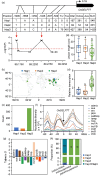
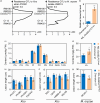
Lipids and lipid metabolites have essential roles in plant-pathogen interactions. GDSL-type lipases are involved in lipid metabolism modulating lipid homeostasis. Some plant GDSLs modulate lipid metabolism altering hormone signal transduction to regulate host-defence immunity. Here, we functionally characterized a rice lipase, OsGELP77, promoting both immunity and yield. OsGELP77 expression was induced by pathogen infection and jasmonic acid (JA) treatment. Overexpression of OsGELP77 enhanced rice resistance to both bacterial and fungal pathogens, while loss-of-function of osgelp77 showed susceptibility. OsGELP77 localizes to endoplasmic reticulum and is a functional lipase hydrolysing universal lipid substrates. Lipidomics analyses demonstrate that OsGELP77 is crucial for lipid metabolism and lipid-derived JA homeostasis. Genetic analyses confirm that OsGELP77-modulated resistance depends on JA signal transduction. Moreover, population genetic analyses indicate that OsGELP77 expression level is positively correlated with rice resistance against pathogens. Three haplotypes were classified based on nucleotide polymorphisms in the OsGELP77 promoter where OsGELP77Hap3 is an elite haplotype. Three OsGELP77 haplotypes are differentially distributed in wild and cultivated rice, while OsGELP77Hap3 has been broadly pyramided for hybrid rice development. Furthermore, quantitative trait locus (QTL) mapping and resistance evaluation of the constructed near-isogenic line validated OsGELP77, a QTL for broad-spectrum disease resistance. In addition, OsGELP77-modulated lipid metabolism promotes JA accumulation facilitating grain yield. Notably, the hub defence regulator OsWRKY45 acts upstream of OsGELP77 by initiating the JA-dependent signalling to trigger immunity. Together, OsGELP77, a QTL contributing to immunity and yield, is a candidate for breeding broad-spectrum resistant and high-yielding rice.
Keywords: OsGELP77; OsWRKY45; QTL; disease resistance; lipase; rice.
© 2023 The Authors. Plant Biotechnology Journal published by Society for Experimental Biology and The Association of Applied Biologists and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Akoh, C.C. , Lee, G.C. , Liaw, Y.C. , Huang, T.H. and Shaw, J.F. (2004) GDSL family of serine esterases/lipases. Prog. Lipid Res. 43, 534–552. - PubMed
-
- Brick, D.J. , Brumlik, M.J. , Buckley, J.T. , Cao, J.X. , Davies, P.C. , Misra, S. , Tranbarger, T.J. et al. (1995) A new family of lipolytic plant enzymes with members in rice, arabidopsis and maize. FEBS Lett. 377, 475–480. - PubMed
-
- Brodersen, P. , Petersen, M. , Bjørn Nielsen, H. , Zhu, S. , Newman, M.A. , Shokat, K.M. , Rietz, S. et al. (2006) Arabidopsis MAP kinase 4 regulates salicylic acid‐ and jasmonic acid/ethylene‐dependent responses via EDS1 and PAD4. Plant J. 47, 532–546. - PubMed
-
- Cai, Q. , Yuan, Z. , Chen, M. , Yin, C. , Luo, Z. , Zhao, X. , Liang, W. et al. (2014) Jasmonic acid regulates spikelet development in rice. Nat. Commun. 5, 3476. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

