Adverse outcomes and an immunosuppressed endotype in septic patients with reduced IFN-γ ELISpot
- PMID: 38100268
- PMCID: PMC10906237
- DOI: 10.1172/jci.insight.175785
Adverse outcomes and an immunosuppressed endotype in septic patients with reduced IFN-γ ELISpot
Abstract
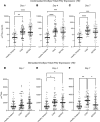
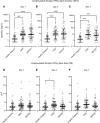
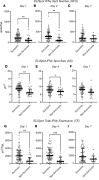
BACKGROUNDSepsis remains a major clinical challenge for which successful treatment requires greater precision in identifying patients at increased risk of adverse outcomes requiring different therapeutic approaches. Predicting clinical outcomes and immunological endotyping of septic patients generally relies on using blood protein or mRNA biomarkers, or static cell phenotyping. Here, we sought to determine whether functional immune responsiveness would yield improved precision.METHODSAn ex vivo whole-blood enzyme-linked immunosorbent spot (ELISpot) assay for cellular production of interferon γ (IFN-γ) was evaluated in 107 septic and 68 nonseptic patients from 5 academic health centers using blood samples collected on days 1, 4, and 7 following ICU admission.RESULTSCompared with 46 healthy participants, unstimulated and stimulated whole-blood IFN-γ expression was either increased or unchanged, respectively, in septic and nonseptic ICU patients. However, in septic patients who did not survive 180 days, stimulated whole-blood IFN-γ expression was significantly reduced on ICU days 1, 4, and 7 (all P < 0.05), due to both significant reductions in total number of IFN-γ-producing cells and amount of IFN-γ produced per cell (all P < 0.05). Importantly, IFN-γ total expression on days 1 and 4 after admission could discriminate 180-day mortality better than absolute lymphocyte count (ALC), IL-6, and procalcitonin. Septic patients with low IFN-γ expression were older and had lower ALCs and higher soluble PD-L1 and IL-10 concentrations, consistent with an immunosuppressed endotype.CONCLUSIONSA whole-blood IFN-γ ELISpot assay can both identify septic patients at increased risk of late mortality and identify immunosuppressed septic patients.TRIAL REGISTRYN/A.FUNDINGThis prospective, observational, multicenter clinical study was directly supported by National Institute of General Medical Sciences grant R01 GM-139046, including a supplement (R01 GM-139046-03S1) from 2022 to 2024.
Keywords: Adaptive immunity; Cellular immune response; Immunology; T cells.
Conflict of interest statement
Figures

Update of
-
Adverse Long-Term Outcomes and an Immune Suppressed Endotype in Sepsis Patients with Reduced Interferon-γELISpot: A Multicenter, Prospective Observational Study.medRxiv [Preprint]. 2023 Sep 24:2023.09.13.23295360. doi: 10.1101/2023.09.13.23295360. medRxiv. 2023. Update in: JCI Insight. 2024 Jan 23;9(2):e175785. doi: 10.1172/jci.insight.175785. PMID: 37745385 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
- R35 GM134880/GM/NIGMS NIH HHS/United States
- T32 GM008721/GM/NIGMS NIH HHS/United States
- R01 GM149657/GM/NIGMS NIH HHS/United States
- RM1 GM139690/GM/NIGMS NIH HHS/United States
- R35 GM126928/GM/NIGMS NIH HHS/United States
- R35 GM133756/GM/NIGMS NIH HHS/United States
- R35 GM142481/GM/NIGMS NIH HHS/United States
- IK6 BX006192/BX/BLRD VA/United States
- R35 GM140806/GM/NIGMS NIH HHS/United States
- R01 GM124156/GM/NIGMS NIH HHS/United States
- R01 GM139046/GM/NIGMS NIH HHS/United States
- R35 GM140881/GM/NIGMS NIH HHS/United States
- R35 GM012698/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials

