Pleural macrophages translocate to the lung during infection to promote improved influenza outcomes
- PMID: 38100417
- PMCID: PMC10743374
- DOI: 10.1073/pnas.2300474120
Pleural macrophages translocate to the lung during infection to promote improved influenza outcomes
Abstract
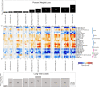
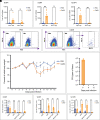
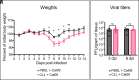
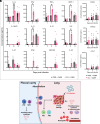
Seasonal influenza results in 3 to 5 million cases of severe disease and 250,000 to 500,000 deaths annually. Macrophages have been implicated in both the resolution and progression of the disease, but the drivers of these outcomes are poorly understood. We probed mouse lung transcriptomic datasets using the Digital Cell Quantifier algorithm to predict immune cell subsets that correlated with mild or severe influenza A virus (IAV) infection outcomes. We identified a unique lung macrophage population that transcriptionally resembled small serosal cavity macrophages and whose presence correlated with mild disease. Until now, the study of serosal macrophage translocation in the context of viral infections has been neglected. Here, we show that pleural macrophages (PMs) migrate from the pleural cavity to the lung after infection with IAV. We found that the depletion of PMs increased morbidity and pulmonary inflammation. There were increased proinflammatory cytokines in the pleural cavity and an influx of neutrophils within the lung. Our results show that PMs are recruited to the lung during IAV infection and contribute to recovery from influenza. This study expands our knowledge of PM plasticity and identifies a source of lung macrophages independent of monocyte recruitment and local proliferation.
Keywords: influenza; macrophages; pleural cavity; tissue deconvolution; transcriptomics.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures



Similar articles
-
PPAR-γ in Macrophages Limits Pulmonary Inflammation and Promotes Host Recovery following Respiratory Viral Infection.J Virol. 2019 Apr 17;93(9):e00030-19. doi: 10.1128/JVI.00030-19. Print 2019 May 1. J Virol. 2019. PMID: 30787149 Free PMC article.
-
The Emerging Role of Pleural Macrophages in Influenza Defense.DNA Cell Biol. 2025;44(3):127-131. doi: 10.1089/dna.2024.0283. Epub 2025 Jan 27. DNA Cell Biol. 2025. PMID: 39868992 Review.
-
Cysteinyl Maresins Reprogram Macrophages to Protect Mice from Streptococcus pneumoniae after Influenza A Virus Infection.mBio. 2022 Aug 30;13(4):e0126722. doi: 10.1128/mbio.01267-22. Epub 2022 Aug 1. mBio. 2022. PMID: 35913160 Free PMC article.
-
Melatonin improves influenza virus infection-induced acute exacerbation of COPD by suppressing macrophage M1 polarization and apoptosis.Respir Res. 2024 Apr 27;25(1):186. doi: 10.1186/s12931-024-02815-0. Respir Res. 2024. PMID: 38678295 Free PMC article.
-
Influenza A virus interactions with macrophages: Lessons from epithelial cells.Cell Microbiol. 2020 May;22(5):e13170. doi: 10.1111/cmi.13170. Epub 2020 Feb 18. Cell Microbiol. 2020. PMID: 31990121 Review.
Cited by
-
Eosinophils and pleural macrophages counter regulate IL-33-elicited airway inflammation via the 12/15-lipoxygenase pathway.Front Immunol. 2025 Apr 17;16:1565670. doi: 10.3389/fimmu.2025.1565670. eCollection 2025. Front Immunol. 2025. PMID: 40313934 Free PMC article.
-
Treatment with lipoxin A4 improves influenza A infection outcome, induces macrophage reprogramming, anti-inflammatory and pro-resolutive responses.Inflamm Res. 2024 Nov;73(11):1903-1918. doi: 10.1007/s00011-024-01939-9. Epub 2024 Aug 30. Inflamm Res. 2024. PMID: 39214890
References
-
- Horimoto T., Kawaoka Y., Influenza: Lessons from past pandemics, warnings from current incidents. Nat. Rev. Microbiol. 3, 591–600 (2005). - PubMed
-
- Short K. R., Kroeze E. J., Fouchier R. A., Kuiken T., Pathogenesis of influenza-induced acute respiratory distress syndrome. Lancet Infect. Dis. 14, 57–69 (2014). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

