A role for mutations in AK9 and other genes affecting ependymal cells in idiopathic normal pressure hydrocephalus
- PMID: 38100419
- PMCID: PMC10743366
- DOI: 10.1073/pnas.2300681120
A role for mutations in AK9 and other genes affecting ependymal cells in idiopathic normal pressure hydrocephalus
Abstract
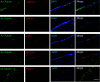
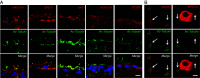
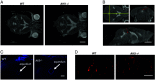
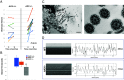
Idiopathic normal pressure hydrocephalus (iNPH) is an enigmatic neurological disorder that develops after age 60 and is characterized by gait difficulty, dementia, and incontinence. Recently, we reported that heterozygous CWH43 deletions may cause iNPH. Here, we identify mutations affecting nine additional genes (AK9, RXFP2, PRKD1, HAVCR1, OTOG, MYO7A, NOTCH1, SPG11, and MYH13) that are statistically enriched among iNPH patients. The encoded proteins are all highly expressed in choroid plexus and ependymal cells, and most have been associated with cilia. Damaging mutations in AK9, which encodes an adenylate kinase, were detected in 9.6% of iNPH patients. Mice homozygous for an iNPH-associated AK9 mutation displayed normal cilia structure and number, but decreased cilia motility and beat frequency, communicating hydrocephalus, and balance impairment. AK9+/- mice displayed normal brain development and behavior until early adulthood, but subsequently developed communicating hydrocephalus. Together, our findings suggest that heterozygous mutations that impair ventricular epithelial function may contribute to iNPH.
Keywords: AK9; motile cilia; normal pressure hydrocephalus; whole exome sequencing.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures



Similar articles
-
Deletions in CWH43 cause idiopathic normal pressure hydrocephalus.EMBO Mol Med. 2021 Mar 5;13(3):e13249. doi: 10.15252/emmm.202013249. Epub 2021 Jan 18. EMBO Mol Med. 2021. PMID: 33459505 Free PMC article.
-
Exploring mechanisms of ventricular enlargement in idiopathic normal pressure hydrocephalus: a role of cerebrospinal fluid dynamics and motile cilia.Fluids Barriers CNS. 2021 Apr 19;18(1):20. doi: 10.1186/s12987-021-00243-6. Fluids Barriers CNS. 2021. PMID: 33874972 Free PMC article. Review.
-
Increased plasmin-mediated proteolysis of L1CAM in a mouse model of idiopathic normal pressure hydrocephalus.Proc Natl Acad Sci U S A. 2021 Aug 17;118(33):e2010528118. doi: 10.1073/pnas.2010528118. Proc Natl Acad Sci U S A. 2021. PMID: 34380733 Free PMC article.
-
[Epidemiology of Idiopathic Normal Pressure Hydrocephalus and Hereditary Hydrocephalus].No Shinkei Geka. 2022 Mar;50(2):309-317. doi: 10.11477/mf.1436204559. No Shinkei Geka. 2022. PMID: 35400649 Japanese.
-
Idiopathic Normal Pressure Hydrocephalus With Stuttering: Report of Two Cases and Review of the Literature.World Neurosurg. 2020 Mar;135:176-179. doi: 10.1016/j.wneu.2019.11.152. Epub 2019 Dec 2. World Neurosurg. 2020. PMID: 31805405 Free PMC article. Review.
Cited by
-
Motile Cilia in Female and Male Reproductive Tracts and Fertility.Cells. 2024 Nov 28;13(23):1974. doi: 10.3390/cells13231974. Cells. 2024. PMID: 39682722 Free PMC article. Review.
-
Mouse radial spoke 3 is a metabolic and regulatory hub in cilia.Nat Struct Mol Biol. 2025 Aug;32(8):1542-1554. doi: 10.1038/s41594-025-01594-6. Epub 2025 Jun 27. Nat Struct Mol Biol. 2025. PMID: 40579595
-
Familial vs sporadic normal pressure hydrocephalus: a comparative study.J Neurol. 2025 Feb 25;272(3):229. doi: 10.1007/s00415-025-12970-z. J Neurol. 2025. PMID: 39998670
-
Multiciliated ependymal cells: an update on biology and pathology in the adult brain.Acta Neuropathol. 2024 Sep 10;148(1):39. doi: 10.1007/s00401-024-02784-0. Acta Neuropathol. 2024. PMID: 39254862 Review.
-
Genetic Risk Factors in Normal Pressure Hydrocephalus: What We Know and What Is Next.Mov Disord. 2025 Jul;40(7):1233-1247. doi: 10.1002/mds.30206. Epub 2025 Apr 23. Mov Disord. 2025. PMID: 40266017 Free PMC article.
References
-
- Adams R. D., Fisher C. M., Hakim S., Ojemann R. G., Sweet W. H., Symptomatic occult hydrocephalus with "normal" cerebrospinal-fluid pressure. A treatable syndrome. N Engl. J. Med. 273, 117–126 (1965). - PubMed
-
- Tanaka N., Yamaguchi S., Ishikawa H., Ishii H., Meguro K., Prevalence of possible idiopathic normal-pressure hydrocephalus in Japan: The Osaki-Tajiri project. Neuroepidemiology 32, 171–175 (2009). - PubMed
-
- Hiraoka K., Meguro K., Mori E., Prevalence of idiopathic normal-pressure hydrocephalus in the elderly population of a Japanese rural community. Neurol. Med. Chir. (Tokyo) 48, 197–199; discussion 199–200 (2008). - PubMed
-
- Martin-Laez R., Caballero-Arzapalo H., Lopez-Menendez L. A., Arango-Lasprilla J. C., Vazquez-Barquero A., Epidemiology of idiopathic normal pressure hydrocephalus: A systematic review of the literature. World Neurosurg. 84, 2002–2009 (2015). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

