Early human lung immune cell development and its role in epithelial cell fate
- PMID: 38100545
- PMCID: PMC7615868
- DOI: 10.1126/sciimmunol.adf9988
Early human lung immune cell development and its role in epithelial cell fate
Abstract
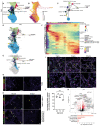
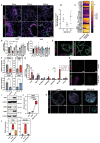
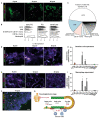
Studies of human lung development have focused on epithelial and mesenchymal cell types and function, but much less is known about the developing lung immune cells, even though the airways are a major site of mucosal immunity after birth. An unanswered question is whether tissue-resident immune cells play a role in shaping the tissue as it develops in utero. Here, we profiled human embryonic and fetal lung immune cells using scRNA-seq, smFISH, and immunohistochemistry. At the embryonic stage, we observed an early wave of innate immune cells, including innate lymphoid cells, natural killer cells, myeloid cells, and lineage progenitors. By the canalicular stage, we detected naive T lymphocytes expressing high levels of cytotoxicity genes and the presence of mature B lymphocytes, including B-1 cells. Our analysis suggests that fetal lungs provide a niche for full B cell maturation. Given the presence and diversity of immune cells during development, we also investigated their possible effect on epithelial maturation. We found that IL-1β drives epithelial progenitor exit from self-renewal and differentiation to basal cells in vitro. In vivo, IL-1β-producing myeloid cells were found throughout the lung and adjacent to epithelial tips, suggesting that immune cells may direct human lung epithelial development.
Conflict of interest statement
Figures

References
-
- Hynds RE, Zacharias WJ, Nikolić MZ, Königshoff M, Eickelberg O, Gosens R, de Coppi P, Janes SM, Morrisey E, Clevers H, Ryan AL, et al. National Heart, lung, and Blood Institute and building respiratory epithelium and tissue for Health (BREATH) consortium workshop report: Moving forward in lung regeneration. Am J Respir Cell Mol Biol. 2021;65:22–29. - PMC - PubMed
-
- Noncommunicable diseases. World Health Organization; 2021. available at https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases.
-
- deMello DE, Reid LM. Embryonic and early fetal development of human lung vasculature and its functional implications. Pediatr Dev Pathol. 2000;3:439–449. - PubMed
Publication types
MeSH terms
Grants and funding
- MR/W025051/1/MRC_/Medical Research Council/United Kingdom
- NC/X001644/1/NC3RS_/National Centre for the Replacement, Refinement and Reduction of Animals in Research/United Kingdom
- MR/S005579/1/MRC_/Medical Research Council/United Kingdom
- EDDPJT-NOV22/100042/CRUK_/Cancer Research UK/United Kingdom
- 206194/WT_/Wellcome Trust/United Kingdom
- MR/S035907/1/MRC_/Medical Research Council/United Kingdom
- MR/S035842/1/MRC_/Medical Research Council/United Kingdom
- 220268/WT_/Wellcome Trust/United Kingdom
- MR/P009581/1/MRC_/Medical Research Council/United Kingdom
- RP-2017-08-ST2-002/DH_/Department of Health/United Kingdom
- MR/W00111X/1/MRC_/Medical Research Council/United Kingdom
- MR/R015635/1/MRC_/Medical Research Council/United Kingdom
- MR/R006237/1/MRC_/Medical Research Council/United Kingdom