Modularized microrobot with lock-and-detachable modules for targeted cell delivery in bile duct
- PMID: 38100592
- PMCID: PMC10848723
- DOI: 10.1126/sciadv.adj0883
Modularized microrobot with lock-and-detachable modules for targeted cell delivery in bile duct
Abstract
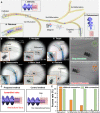
The magnetic microrobots promise benefits in minimally invasive cell-based therapy. However, they generally suffer from an inevitable compromise between their magnetic responsiveness and biomedical functions. Herein, we report a modularized microrobot consisting of magnetic actuation (MA) and cell scaffold (CS) modules. The MA module with strong magnetism and pH-responsive deformability and the CS module with cell loading-release capabilities were fabricated by three-dimensional printing technique. Subsequently, assembly of modules was performed by designing a shaft-hole structure and customizing their relative dimensions, which enabled magnetic navigation in complex environments, while not deteriorating the cellular functionalities. On-demand disassembly at targeted lesion was then realized to facilitate CS module delivery and retrieval of the MA module. Furthermore, the feasibility of proposed system was validated in an in vivo rabbit bile duct. Therefore, this work presents a modular design-based strategy that enables uncompromised fabrication of multifunctional microrobots and stimulates their development for future cell-based therapy.
Figures





References
-
- G. Q. Daley, D. T. Scadden, Prospects for stem cell-based therapy. Cell 132, 544–548 (2008). - PubMed
-
- J. M. Hallett, S. Ferreira-Gonzalez, T. Y. Man, A. M. Kilpatrick, H. Esser, K. Thirlwell, M. T. Macmillan, D. Rodrigo-Torres, B. J. Dwyer, V. L. Gadd, C. Ashmore-Harris, W.-Y. Lu, J. P. Thomson, M. A. Jansen, E. O’Duibhir, P. J. Starkey Lewis, L. Campana, R. E. Aird, T. S. R. Bate, A. R. Fraser, J. D. M. Campbell, G. C. Oniscu, D. C. Hay, A. Callanan, S. J. Forbes, Human biliary epithelial cells from discarded donor livers rescue bile duct structure and function in a mouse model of biliary disease. Cell Stem Cell 29, 355–371.e10 (2022). - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

