Cryo-EM structure of influenza helical nucleocapsid reveals NP-NP and NP-RNA interactions as a model for the genome encapsidation
- PMID: 38100595
- PMCID: PMC10848707
- DOI: 10.1126/sciadv.adj9974
Cryo-EM structure of influenza helical nucleocapsid reveals NP-NP and NP-RNA interactions as a model for the genome encapsidation
Abstract
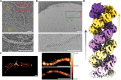
Influenza virus genome encapsidation is essential for the formation of a helical viral ribonucleoprotein (vRNP) complex composed of nucleoproteins (NP), the trimeric polymerase, and the viral genome. Although low-resolution vRNP structures are available, it remains unclear how the viral RNA is encapsidated and how NPs assemble into the helical filament specific of influenza vRNPs. In this study, we established a biological tool, the RNP-like particles assembled from recombinant influenza A virus NP and synthetic RNA, and we present the first subnanometric cryo-electron microscopy structure of the helical NP-RNA complex (8.7 to 5.3 Å). The helical RNP-like structure reveals a parallel double-stranded conformation, allowing the visualization of NP-NP and NP-RNA interactions. The RNA, located at the interface of neighboring NP protomers, interacts with conserved residues previously described as essential for the NP-RNA interaction. The NP undergoes conformational changes to enable RNA binding and helix formation. Together, our findings provide relevant insights for understanding the mechanism for influenza genome encapsidation.
Figures



Similar articles
-
Influenza a virus antiparallel helical nucleocapsid-like pseudo-atomic structure.Nucleic Acids Res. 2025 Jan 24;53(3):gkae1211. doi: 10.1093/nar/gkae1211. Nucleic Acids Res. 2025. PMID: 39673795 Free PMC article.
-
Biochemical and structural evidence in support of a coherent model for the formation of the double-helical influenza A virus ribonucleoprotein.mBio. 2012 Dec 26;4(1):e00467-12. doi: 10.1128/mBio.00467-12. mBio. 2012. PMID: 23269829 Free PMC article.
-
Ultrastructure of influenza virus ribonucleoprotein complexes during viral RNA synthesis.Commun Biol. 2021 Jul 9;4(1):858. doi: 10.1038/s42003-021-02388-4. Commun Biol. 2021. PMID: 34244608 Free PMC article.
-
Filovirus helical nucleocapsid structures.Microscopy (Oxf). 2023 Jun 8;72(3):178-190. doi: 10.1093/jmicro/dfac049. Microscopy (Oxf). 2023. PMID: 36242583 Review.
-
A structural understanding of influenza virus genome replication.Trends Microbiol. 2023 Mar;31(3):308-319. doi: 10.1016/j.tim.2022.09.015. Epub 2022 Nov 3. Trends Microbiol. 2023. PMID: 36336541 Review.
Cited by
-
Analysis of polyclonal and monoclonal antibody to the influenza virus nucleoprotein in different oligomeric states.Virus Res. 2025 May;355:199563. doi: 10.1016/j.virusres.2025.199563. Epub 2025 Mar 24. Virus Res. 2025. PMID: 40139568 Free PMC article.
-
Functionality of IAV packaging signals depends on site-specific charges within the viral nucleoprotein.J Virol. 2024 Apr 16;98(4):e0197223. doi: 10.1128/jvi.01972-23. Epub 2024 Mar 12. J Virol. 2024. PMID: 38470155 Free PMC article.
-
The Influenza A Virus Replication Cycle: A Comprehensive Review.Viruses. 2024 Feb 19;16(2):316. doi: 10.3390/v16020316. Viruses. 2024. PMID: 38400091 Free PMC article. Review.
-
Molecular basis of influenza ribonucleoprotein complex assembly and processive RNA synthesis.Science. 2025 May 15;388(6748):eadq7597. doi: 10.1126/science.adq7597. Epub 2025 May 15. Science. 2025. PMID: 40373132
-
Structure of the tilapia lake virus nucleoprotein bound to RNA.Nucleic Acids Res. 2025 Feb 8;53(4):gkaf112. doi: 10.1093/nar/gkaf112. Nucleic Acids Res. 2025. PMID: 39995042 Free PMC article.
References
-
- R. M. Pinto, S. Bakshi, S. Lytras, M. K. Zakaria, S. Swingler, J. C. Worrell, V. Herder, K. E. Hargrave, M. Varjak, N. Cameron-Ruiz, M. Collados Rodriguez, M. Varela, A. Wickenhagen, C. Loney, Y. Pei, J. Hughes, E. Valette, M. L. Turnbull, W. Furnon, Q. Gu, L. Orr, A. Taggart, O. Diebold, C. Davis, C. Boutell, F. Grey, E. Hutchinson, P. Digard, I. Monne, S. K. Wootton, M. K. L. MacLeod, S. J. Wilson, M. Palmarini, BTN3A3 evasion promotes the zoonotic potential of influenza A viruses. Nature 619, 338–347 (2023). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

