Efficacy assessment of an active tau immunotherapy in Alzheimer's disease patients with amyloid and tau pathology: a post hoc analysis of the "ADAMANT" randomised, placebo-controlled, double-blind, multi-centre, phase 2 clinical trial
- PMID: 38101301
- PMCID: PMC10733085
- DOI: 10.1016/j.ebiom.2023.104923
Efficacy assessment of an active tau immunotherapy in Alzheimer's disease patients with amyloid and tau pathology: a post hoc analysis of the "ADAMANT" randomised, placebo-controlled, double-blind, multi-centre, phase 2 clinical trial
Abstract
Background: Tau pathology correlates with and predicts clinical decline in Alzheimer's disease. Approved tau-targeted therapies are not available.
Methods: ADAMANT, a 24-month randomised, placebo-controlled, parallel-group, double-blinded, multicenter, Phase 2 clinical trial (EudraCT2015-000630-30, NCT02579252) enrolled 196 participants with Alzheimer's disease; 119 are included in this post-hoc subgroup analysis. AADvac1, active immunotherapy against pathological tau protein. A machine learning model predicted likely Amyloid+Tau+ participants from baseline MRI.
Statistical methods: MMRM for change from baseline in cognition, function, and neurodegeneration; linear regression for associations between antibody response and endpoints.
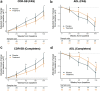
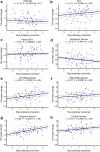
Results: The prediction model achieved PPV of 97.7% for amyloid, 96.2% for tau. 119 participants in the full analysis set (70 treatment and 49 placebo) were classified as A+T+. A trend for CDR-SB 104-week change (estimated marginal means [emm] = -0.99 points, 95% CI [-2.13, 0.13], p = 0.0825]) and ADCS-MCI-ADL (emm = 3.82 points, CI [-0.29, 7.92], p = 0.0679) in favour of the treatment group was seen. Reduction was seen in plasma NF-L (emm = -0.15 log pg/mL, CI [-0.27, -0.03], p = 0.0139). Higher antibody response to AADvac1 was related to slowing of decline on CDR-SB (rho = -0.10, CI [-0.21, 0.01], p = 0.0376) and ADL (rho = 0.15, CI [0.03, 0.27], p = 0.0201), and related to slower brain atrophy (rho = 0.18-0.35, p < 0.05 for temporal volume, whole cortex, and right and left hippocampus).
Conclusions: In the subgroup of ML imputed or CSF identified A+T+, AADvac1 slowed AD-related decline in an antibody-dependent manner. Larger anti-tau trials are warranted.
Funding: AXON Neuroscience SE.
Keywords: Alzheimer’s disease; Immunotherapy; Machine learning; Post-hoc analysis; Tau.
Copyright © 2023 AXON NEUROSCIENCE SE. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests Nick Cullen received personal fees from AXON Neuroscience SE. All authors affiliated with AXON NEUROSCIENCE SE or one of its subsidiaries received salary from their respective companies. Jozef Hanes, Eva Kontsekova, and Branislav Kovacech report patents with AXON Neuroscience R&D Services SE. Petr Novak received payments from F. Hoffmann-La Roche AG. The investigators’ institutions received reimbursement on a per-patient per-visit basis. Duygu Tosun’s institution received payments from AXON Neuroscience for image processing, and payments to the institution from Siemens Medical Solutions USA, Inc., Takeda Pharmaceutical Company Ltd., DOD WW81XWH-19-1-0669, NIH/NIA U19AG024904, NIH/NIA U01AG068057, NIH/NIA U24AG074855, NIH/NIA R01AG058676. Reinhold Schmidt has received personal fees and honoraria for image analyses from AXON NEUROSCIENCE. Stefan Ropele reports no conflict of interest. Bengt Winblad reports personal fees for taking part in Scientific Advisory Board meetings and Data Safety Management Board meetings from AXON NEUROSCIENCE, and from Alzinova DSMB and Artery TX SAB. Dr. Feldman reports a service agreement between Axon Neuroscience and UCSD for consulting and travel with all payments to UCSD and no personal funds received. Other activities to report include: grant funding from Annovis (QR Pharma), Vivoryon (Probiodrug), AC Immune, and LuMind; service agreements for consulting activities with LuMind, Genentech (DSMB), Roche/Banner (DMC), Tau Consortium (SAB), Samus Therapeutics, Biosplice Therapeutics, Novo Nordisk Inc., Janssen Research & Development LLC, and Arrowhead Pharmaceuticals with no personal funds received and all payments to UCSD. He also reports a philanthropic donation to UCSD from the Epstein Family Alzheimer's Disease Collaboration for therapeutic research in AD.
Figures


Similar articles
-
Post hoc analysis of ADAMANT, a phase 2 clinical trial of active tau immunotherapy with AADvac1 in patients with Alzheimer's disease, positive for plasma p-tau217.Alzheimers Res Ther. 2024 Nov 23;16(1):254. doi: 10.1186/s13195-024-01620-7. Alzheimers Res Ther. 2024. PMID: 39580468 Free PMC article. Clinical Trial.
-
ADAMANT: a placebo-controlled randomized phase 2 study of AADvac1, an active immunotherapy against pathological tau in Alzheimer's disease.Nat Aging. 2021 Jun;1(6):521-534. doi: 10.1038/s43587-021-00070-2. Epub 2021 Jun 14. Nat Aging. 2021. PMID: 37117834 Clinical Trial.
-
FUNDAMANT: an interventional 72-week phase 1 follow-up study of AADvac1, an active immunotherapy against tau protein pathology in Alzheimer's disease.Alzheimers Res Ther. 2018 Oct 24;10(1):108. doi: 10.1186/s13195-018-0436-1. Alzheimers Res Ther. 2018. PMID: 30355322 Free PMC article. Clinical Trial.
-
Tau biomarkers in Alzheimer's disease: towards implementation in clinical practice and trials.Lancet Neurol. 2022 Aug;21(8):726-734. doi: 10.1016/S1474-4422(22)00168-5. Epub 2022 May 25. Lancet Neurol. 2022. PMID: 35643092 Review.
-
Understanding disease progression and improving Alzheimer's disease clinical trials: Recent highlights from the Alzheimer's Disease Neuroimaging Initiative.Alzheimers Dement. 2019 Jan;15(1):106-152. doi: 10.1016/j.jalz.2018.08.005. Epub 2018 Oct 13. Alzheimers Dement. 2019. PMID: 30321505 Review.
Cited by
-
Advancing Nanomaterial-Based Strategies for Alzheimer's Disease: A Perspective.JACS Au. 2025 Mar 31;5(4):1519-1537. doi: 10.1021/jacsau.5c00002. eCollection 2025 Apr 28. JACS Au. 2025. PMID: 40313833 Free PMC article. Review.
-
pS396/pS404 (PHF1) tau vaccine outperforms pS199/pS202 (AT8) in rTg4510 tauopathy model.NPJ Vaccines. 2025 May 13;10(1):94. doi: 10.1038/s41541-025-01147-4. NPJ Vaccines. 2025. PMID: 40360566 Free PMC article.
-
Novel Therapeutic Strategies in Alzheimer's Disease: Pitfalls and Challenges of Anti-Amyloid Therapies and Beyond.J Clin Med. 2024 May 25;13(11):3098. doi: 10.3390/jcm13113098. J Clin Med. 2024. PMID: 38892809 Free PMC article. Review.
-
Insights into the use of biomarkers in clinical trials in Alzheimer's disease.EBioMedicine. 2024 Oct;108:105322. doi: 10.1016/j.ebiom.2024.105322. Epub 2024 Oct 3. EBioMedicine. 2024. PMID: 39366844 Free PMC article. Review.
-
Newer Therapeutic Approaches in Treating Alzheimer's Disease: A Comprehensive Review.ACS Omega. 2025 Feb 3;10(6):5148-5171. doi: 10.1021/acsomega.4c05527. eCollection 2025 Feb 18. ACS Omega. 2025. PMID: 39989768 Free PMC article. Review.
References
-
- Kontsekova E., Zilka N., Kovacech B., Novak P., Novak M. First-in-man tau vaccine targeting structural determinants essential for pathological tau-tau interaction reduces tau oligomerisation and neurofibrillary degeneration in an Alzheimer's disease model. Alzheimers Res Ther. 2014;6(4):44. - PMC - PubMed
-
- Novak P., Kovacech B., Katina S., et al. ADAMANT: a placebo-controlled randomized phase 2 study of AADvac1, an active immunotherapy against pathological tau in Alzheimer’s disease. Nat Aging. 2021;1(6):521–534. - PubMed
-
- Tosun D., Chen Y.F., Yu P., et al. Amyloid status imputed from a multimodal classifier including structural MRI distinguishes progressors from nonprogressors in a mild Alzheimer's disease clinical trial cohort. Alzheimers Dement. 2016;12(9):977–986. - PubMed

