The blink reflex and its modulation - Part 1: Physiological mechanisms
- PMID: 38102022
- PMCID: PMC10978309
- DOI: 10.1016/j.clinph.2023.11.015
The blink reflex and its modulation - Part 1: Physiological mechanisms
Erratum in
-
Erratum to "The blink reflex and its modulation - Part 1: Physiological mechanisms" [Clin. Neurophysiol. 160 (2024) 130-152].Clin Neurophysiol. 2024 Jul;163:292. doi: 10.1016/j.clinph.2024.03.026. Epub 2024 Apr 10. Clin Neurophysiol. 2024. PMID: 38604903 Free PMC article. No abstract available.
Abstract
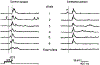
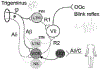
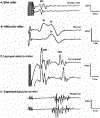
The blink reflex (BR) is a protective eye-closure reflex mediated by brainstem circuits. The BR is usually evoked by electrical supraorbital nerve stimulation but can be elicited by a variety of sensory modalities. It has a long history in clinical neurophysiology practice. Less is known, however, about the many ways to modulate the BR. Various neurophysiological techniques can be applied to examine different aspects of afferent and efferent BR modulation. In this line, classical conditioning, prepulse and paired-pulse stimulation, and BR elicitation by self-stimulation may serve to investigate various aspects of brainstem connectivity. The BR may be used as a tool to quantify top-down modulation based on implicit assessment of the value of blinking in a given situation, e.g., depending on changes in stimulus location and probability of occurrence. Understanding the role of non-nociceptive and nociceptive fibers in eliciting a BR is important to get insight into the underlying neural circuitry. Finally, the use of BRs and other brainstem reflexes under general anesthesia may help to advance our knowledge of the brainstem in areas not amenable in awake intact humans. This review summarizes talks held by the Brainstem Special Interest Group of the International Federation of Clinical Neurophysiology at the International Congress of Clinical Neurophysiology 2022 in Geneva, Switzerland, and provides a state-of-the-art overview of the physiology of BR modulation. Understanding the principles of BR modulation is fundamental for a valid and thoughtful clinical application (reviewed in part 2) (Gunduz et al., submitted).
Keywords: Anesthesia; Classical conditioning; Pain; Peripersonal space; Prepulse inhibition; Protective reflex.
Copyright © 2023 International Federation of Clinical Neurophysiology. All rights reserved.
Conflict of interest statement
Conflict of interest MH: Relevant: none. Irrelevant: MH gets a share of patent royalties given to NIH by Brainsway for licensing a patent on the H-coil, a device for magnetic stimulation. He serves on the Medical Advisory Board of Brainsway, QuantalX and VoxNeuro. He has consulted for Janssen Pharmaceutical. None of the remaining authors has any conflict of interest to declare.
Figures
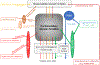

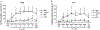

Similar articles
-
A neurophysiological approach to brainstem reflexes. Blink reflex.Neurophysiol Clin. 1999 Feb;29(1):7-38. doi: 10.1016/S0987-7053(99)80039-2. Neurophysiol Clin. 1999. PMID: 10093816 Review.
-
Differential modulation of prepulse inhibition of the blink reflex in peripersonal versus extrapersonal space.Neurophysiol Clin. 2018 Jun;48(3):181-185. doi: 10.1016/j.neucli.2018.03.002. Epub 2018 Apr 7. Neurophysiol Clin. 2018. PMID: 29631777
-
Cortical modulation of brainstem circuits is abnormal in cervical dystonia.Neurosci Lett. 2018 Jun 11;677:84-87. doi: 10.1016/j.neulet.2018.04.047. Epub 2018 Apr 25. Neurosci Lett. 2018. PMID: 29704573
-
In the spotlight: How the brainstem modulates information flow.Clin Neurophysiol. 2023 Apr;148:52-64. doi: 10.1016/j.clinph.2023.01.013. Epub 2023 Feb 6. Clin Neurophysiol. 2023. PMID: 36801494
-
The blink reflex and its modulation - Part 2: Pathophysiology and clinical utility.Clin Neurophysiol. 2024 Apr;160:75-94. doi: 10.1016/j.clinph.2024.02.006. Epub 2024 Feb 9. Clin Neurophysiol. 2024. PMID: 38412746 Review.
Cited by
-
Lethal Interactions of neuronal networks in epilepsy mediated by both synaptic and volume transmission indicate approaches to prevention.Prog Neurobiol. 2025 Jun;249:102770. doi: 10.1016/j.pneurobio.2025.102770. Epub 2025 Apr 19. Prog Neurobiol. 2025. PMID: 40258456 Review.
-
Ultraviolet B Treatment of the Forearm Alters Supraspinal Nociceptive Processing.Pain Res Manag. 2025 Jul 16;2025:6601529. doi: 10.1155/prm/6601529. eCollection 2025. Pain Res Manag. 2025. PMID: 40703500 Free PMC article.
-
Contralateral R1 response in blink reflex in patients with amyotrophic lateral sclerosis.Clin Neurophysiol Pract. 2025 Feb 22;10:47-51. doi: 10.1016/j.cnp.2025.02.005. eCollection 2025. Clin Neurophysiol Pract. 2025. PMID: 40092496 Free PMC article.
-
Clinical Neurophysiological Study for the Diagnosis of Functional Cranial-Cervical Dystonia.Eur J Neurol. 2025 Aug;32(8):e70287. doi: 10.1111/ene.70287. Eur J Neurol. 2025. PMID: 40781931 Free PMC article.
References
-
- Accornero N, Berardelli A, Bini G, Cruccu G, Manfredi M, Caruso R, et al. [Method of recording the corneal reflex in humans, using physiological stimulation]. Boll Soc Ital Biol Sper 1978;54(3):249–51. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

