Reaching the Tumor: Mobility of Polymeric Micelles Inside an In Vitro Tumor-on-a-Chip Model with Dual ECM
- PMID: 38102079
- PMCID: PMC10755695
- DOI: 10.1021/acsami.3c12798
Reaching the Tumor: Mobility of Polymeric Micelles Inside an In Vitro Tumor-on-a-Chip Model with Dual ECM
Abstract
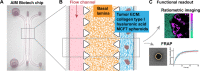
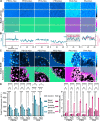
Degradable polymeric micelles are promising drug delivery systems due to their hydrophobic core and responsive design. When applying micellar nanocarriers for tumor delivery, one of the bottlenecks encountered in vivo is the tumor tissue barrier: crossing the dense mesh of cells and the extracellular matrix (ECM). Sometimes overlooked, the extracellular matrix can trap nanoformulations based on charge, size, and hydrophobicity. Here, we used a simple design of a microfluidic chip with two types of ECM and MCF7 spheroids to allow "high-throughput" screening of the interactions between biological interfaces and polymeric micelles. To demonstrate the applicability of the chip, a small library of fluorescently labeled polymeric micelles varying in their hydrophilic shell and hydrophobic core forming blocks was studied. Three widely used hydrophilic shells were tested and compared, namely, poly(ethylene glycol), poly(2-ethyl-2-oxazoline), and poly(acrylic acid), along with two enzymatically degradable dendritic hydrophobic cores (based on hexyl or nonyl end groups). Using ratiometric imaging of unimer:micelle fluorescence and FRAP inside the chip model, we obtained the local assembly state and dynamics inside the chip. Notably, we observed different micelle behaviors in the basal lamina ECM, from avoidance of the ECM structure to binding of the poly(acrylic acid) formulations. Binding to the basal lamina correlated with higher uptake into MCF7 spheroids. Overall, we proposed a simple microfluidic chip containing dual ECM and spheroids for the assessment of the interactions of polymeric nanocarriers with biological interfaces and evaluating nanoformulations' capacity to cross the tumor tissue barrier.
Keywords: extracellular matrix; microfluidics; nanoparticle mobility; polymeric micelles; tumor-on-a-chip.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Pozzi S.; Scomparin A.; Israeli Dangoor S.; Rodriguez Ajamil D.; Ofek P.; Neufeld L.; Krivitsky A.; Vaskovich-Koubi D.; Kleiner R.; Dey P.; Koshrovski-Michael S.; Reisman N.; Satchi-Fainaro R. Meet Me Halfway: Are in Vitro 3D Cancer Models on the Way to Replace in Vivo Models for Nanomedicine Development?. Adv. Drug Delivery Rev. 2021, 175, 11376010.1016/j.addr.2021.04.001. - DOI - PubMed
-
- New Nanomaterials and Techniques for Tumor-Targeted Systems; Huang R.; Wang Y., Eds.; Springer Singapore: Singapore, 2020. 10.1007/978-981-15-5159-8. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

