Enhanced anti-tumor activity of transferrin/folate dual-targeting magnetic nanoparticles using chemo-thermo therapy on retinoblastoma cancer cells Y79
- PMID: 38102193
- PMCID: PMC10724238
- DOI: 10.1038/s41598-023-49171-5
Enhanced anti-tumor activity of transferrin/folate dual-targeting magnetic nanoparticles using chemo-thermo therapy on retinoblastoma cancer cells Y79
Abstract
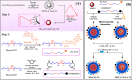
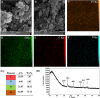
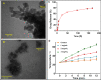
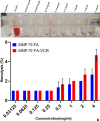
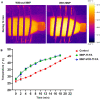
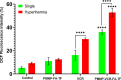
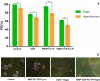
Malignant neoplasms are one of the main causes of death, especially in children, on a global scale, despite strenuous efforts made at advancing both diagnostic and therapeutic modalities. In this regard, a new nanocarrier Vincristine (VCR)-loaded Pluronic f127 polymer-coated magnetic nanoparticles conjugated with folic acid and transferrin (PMNP-VCR-FA-TF) were synthesized and characterized by various methods. The cytotoxicity of these nanoparticles was evaluated in vitro and ex vivo conditions. The in vitro anti-tumor effect of the nanoparticles was evaluated by colony formation assay (CFA) and reactive oxygen species (ROS) in Y79 cell line. The results showed that nanoparticles with two ligands conferred greater toxicity toward Y79 cancer cells than ARPE19 normal cells. Under an alternating magnetic field (AMF), these nanoparticles demonstrated a high specific absorption rate. The CFA and ROS results indicated that the AMF in combination with PMNP-VCR-FA-TF conferred the highest cytotoxicity toward Y79 cells compared with other groups (P < 0.05). PMNP-VCR-FA-TF could play an important role in converting externally applied radiofrequency energy into heat in cancer cells. The present study confirmed that dual targeting chemo-hyperthermia using PMNP-VCR-FA-TF was significantly more effective than hyperthermia or chemotherapy alone, providing a promising platform for precision drug delivery as an essential component in the chemotherapy of retinoblastoma.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

