The centrosomal protein 131 participates in the regulation of mitochondrial apoptosis
- PMID: 38102401
- PMCID: PMC10724242
- DOI: 10.1038/s42003-023-05676-3
The centrosomal protein 131 participates in the regulation of mitochondrial apoptosis
Abstract
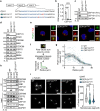
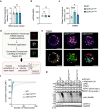
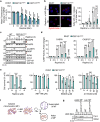
Centriolar satellites are multiprotein aggregates that orbit the centrosome and govern centrosome homeostasis and primary cilia formation. In contrast to the scaffold PCM1, which nucleates centriolar satellites and has been linked to microtubule dynamics, autophagy, and intracellular trafficking, the functions of its interactant CEP131 beyond ciliogenesis remain unclear. Using a knockout strategy in a non-ciliary T-cell line, we report that, although dispensable for centriolar satellite assembly, CEP131 participates in optimal tubulin glycylation and polyglutamylation, and microtubule regrowth. Our unsupervised label-free proteomic analysis by quantitative mass spectrometry further uncovered mitochondrial and apoptotic signatures. CEP131-deficient cells showed an elongated mitochondrial network. Upon cell death inducers targeting mitochondria, knockout cells displayed delayed cytochrome c release from mitochondria, subsequent caspase activation, and apoptosis. This mitochondrial permeabilization defect was intrinsic, and replicable in vitro with isolated organelles. These findings extend CEP131 functions to life-and-death decisions and propose ways to interfere with mitochondrial apoptosis.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Polo-like kinase 4 maintains centriolar satellite integrity by phosphorylation of centrosomal protein 131 (CEP131).J Biol Chem. 2019 Apr 19;294(16):6531-6549. doi: 10.1074/jbc.RA118.004867. Epub 2019 Feb 25. J Biol Chem. 2019. PMID: 30804208 Free PMC article.
-
The centriolar satellite protein CCDC66 interacts with CEP290 and functions in cilium formation and trafficking.J Cell Sci. 2017 Apr 15;130(8):1450-1462. doi: 10.1242/jcs.196832. Epub 2017 Feb 24. J Cell Sci. 2017. PMID: 28235840 Free PMC article.
-
Centriolar satellites expedite mother centriole remodeling to promote ciliogenesis.Elife. 2023 Feb 15;12:e79299. doi: 10.7554/eLife.79299. Elife. 2023. PMID: 36790165 Free PMC article.
-
Centriolar satellites: key mediators of centrosome functions.Cell Mol Life Sci. 2015 Jan;72(1):11-23. doi: 10.1007/s00018-014-1711-3. Epub 2014 Aug 31. Cell Mol Life Sci. 2015. PMID: 25173771 Free PMC article. Review.
-
Regulation of centriolar satellite integrity and its physiology.Cell Mol Life Sci. 2017 Jan;74(2):213-229. doi: 10.1007/s00018-016-2315-x. Epub 2016 Aug 2. Cell Mol Life Sci. 2017. PMID: 27484406 Free PMC article. Review.
Cited by
-
Necrosulfonamide causes oxidation of PCM1 and impairs ciliogenesis and autophagy.iScience. 2024 Mar 26;27(4):109580. doi: 10.1016/j.isci.2024.109580. eCollection 2024 Apr 19. iScience. 2024. PMID: 38600973 Free PMC article.
-
Raptinal: a powerful tool for rapid induction of apoptotic cell death.Cell Death Discov. 2024 Aug 21;10(1):371. doi: 10.1038/s41420-024-02120-1. Cell Death Discov. 2024. PMID: 39164225 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

