An alternative fully human anti-BCMA CAR-T shows response for relapsed or refractory multiple myeloma with anti-BCMA CAR-T exposures previously
- PMID: 38102463
- PMCID: PMC10940153
- DOI: 10.1038/s41417-023-00712-0
An alternative fully human anti-BCMA CAR-T shows response for relapsed or refractory multiple myeloma with anti-BCMA CAR-T exposures previously
Abstract
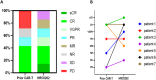
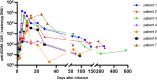
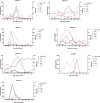
Chimeric antigen receptor T (CAR-T) cells therapy has made remarkable progress in relapsed/refractory multiple myeloma (R/R MM) treatment. Unfortunately, patients still eventually experience disease progression or relapse even after receiving anti-BCMA CAR-T therapy. At present, there are limited data on available treatment options for patients who have progressed on anti-BCMA CAR-T therapy. In this study, we evaluated the safety and efficacy of fully human anti-BCMA CAR-T (HRC0202) in seven R/R MM patients who were previously exposed to anti-BCMA CAR-T therapy. Three patients received 6.0 × 106 CAR+T cells/kg, one patient received 10.0 × 106 CAR+T cells/kg and three patients received 15.0 × 106 CAR+T cells/kg. Cytokine release syndrome (CRS) of grades 1-2 occurred in three patients (42.9%) and grade ≥3 in two patients (28.6%). Immune effector cell-associated neurotoxic syndrome (ICANS) was not observed in any of the patients. The best overall response rate (ORR) was 71.4% (5/7), with a stringent complete response/complete response (sCR/CR) achieved in three patients. The median progression-free survival (PFS) was 269 days, and median overall survival (OS) for all patients was not reached. The median peak concentration (Cmax) of HRC0202 was 30117.70 (range, 6084.35-147415.10) copies/μg DNA. This study indicated that fully human anti-BCMA CAR-T (HRC0202) is a promising treatment for R/R MM patients who relapsed or refractory from prior anti-BCMA CAR-T infusion.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Derudas D, Capraro F, Martinelli G, Cerchione C. Old and new generation immunomodulatory drugs in multiple myeloma. Panminerva Med. 2020;62:207–19. - PubMed
MeSH terms
Substances
Grants and funding
- 20201BBG71009/Jiangxi Provincial Department of Science and Technology (Department of Science and Technology, Jiangxi Province)
- 20212BDH80014/Jiangxi Provincial Department of Science and Technology (Department of Science and Technology, Jiangxi Province)
- 23S11905500/Shanghai Science and Technology Development Foundation (Shanghai Science and Technology Development Fund)
LinkOut - more resources
Full Text Sources
Medical
Research Materials

