"In House" assays for the quantification of Annexin V and its autoantibodies in patients with recurrent pregnancy loss and in vitro fertilisation failures
- PMID: 38102468
- PMCID: PMC10724132
- DOI: 10.1038/s41598-023-49768-w
"In House" assays for the quantification of Annexin V and its autoantibodies in patients with recurrent pregnancy loss and in vitro fertilisation failures
Abstract
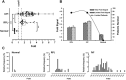
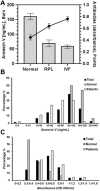
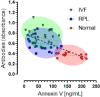
Several studies have been shown that Annexin V (ANXV) autoantibodies concentrations are associated with both early recurrent pregnancy losses (RPLs) or in vitro fertilization failure (IVFf). We investigated the association between ANXV autoantibodies and ANVX levels in RPL, IVFf and normal group women. The study was conducted on 22 female patients with RPLs, 66 patients with IVFf, and 16 normal samples from women who had given birth. ANXV autoantibodies were measured using an ELISA test developed by fixing a homemade recombinant ANXV protein and examined with labeled human antibodies, while ANXV concentrations were measured by a competitive ELISA using a homemade anti ANXV polyclonal antibody. The results showed a clear relationship between the high levels of ANXV autoantibodies and the recurrent abortion. On the other hand, ANXV measurement in those patients showed decreased concentrations compared to normal samples. Negative correlation between ANXV and its autoantibodies levels was reported in almost all patients' samples. Our data supports the possibility that ANXV autoantibodies are a risk factor for reproductive failures associated with both RPLs and/or IVFf and the significant role for ANXV in the maintenance of pregnancy.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

