Efficacy and safety of eptinezumab in patients with chronic migraine and medication-overuse headache: a randomized, double-blind, placebo-controlled study
- PMID: 38102535
- PMCID: PMC10722704
- DOI: 10.1186/s12883-023-03477-z
Efficacy and safety of eptinezumab in patients with chronic migraine and medication-overuse headache: a randomized, double-blind, placebo-controlled study
Abstract
Background: For some people with migraine, despite taking greater amounts of acute headache medication (AHM), they develop an increase in monthly headache days. This cycle of increasing headache days, and in turn AHM use, can lead to a secondary headache disorder called medication-overuse headache (MOH). Preventive medications can prevent migraine from occurring and reduce reliance on AHMs, thereby preventing the cycle of MOH. This study was performed to evaluate the efficacy and safety of eptinezumab to prevent migraine/headache in a mainly Asian patient population with a dual diagnosis of chronic migraine and MOH.
Methods: SUNLIGHT was a phase 3, multicenter, double-blind, parallel-group, placebo-controlled trial. Patients aged 18-75 years with ≥ 8 migraine days/month and a diagnosis of MOH were randomly allocated (1:1) to one of two treatment groups: eptinezumab 100 mg or placebo. Monthly migraine days (MMDs) were captured using a daily electronic diary; the change from baseline in the number of MMDs over Weeks 1-12 was the primary efficacy endpoint.
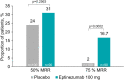
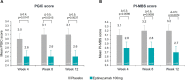
Results: Patients were randomized to eptinezumab 100 mg (n = 93) or placebo (n = 100). Over Weeks 1-12, eptinezumab reduced mean MMDs more than placebo (difference between treatments was -1.2; p = 0.1484). Differences between treatment groups with p-values below 0.05 favoring eptinezumab were observed in 3 out of the 6 key secondary endpoints.
Conclusion: All endpoints numerically favored eptinezumab treatment when compared to placebo; however, this study did not meet its primary endpoint and is therefore negative. No new safety signals were identified in this study, like previous reports that confirmed the safety and tolerability of eptinezumab treatment.
Trial registration: ClinicalTrials.gov identifier: NCT04772742 (26/02/2021).
Keywords: Anti-CGRP; Chronic migraine; Eptinezumab; Medication-overuse headache; Preventive migraine treatment.
© 2023. The Author(s).
Conflict of interest statement
SY, JZ, GL, and ZX disclose no conflicts of interest. AE, GJ, KR, and IF are full-time employees of H. Lundbeck A/S. PPR reports honoraria as a consultant and participation in the last 3 years in advisory boards for AbbVie, Amgen, Biohaven, Chiesi, Eli Lilly, Lundbeck, Novartis, Pfizer, and Teva Pharmaceuticals; institutional research support from AbbVie, AGAUR, ERA-NET NEURON, Instituto Investigación Carlos III, International Headache Society, Novartis, PERIS, RIS3CAT FEDER, and Teva Pharmaceuticals; being a principle investigator for more than 45 clinical trials (phase II, III, and IV) for the preventive treatment of migraine and other headaches; education projects with AbbVie, Almirall, Chiesi, Eli Lilly, Lundbeck, Medlink, Medscape, Neurodiem, Novartis, and Teva Pharmaceuticals; participation in the Scientific Advisory Board of Lilly Foundation Spain and Honorary Secretary of the International Headache Society; and being an associate editor for
Figures


References
-
- Collaborators GBoDS Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(9995):743–800. doi: 10.1016/s0140-6736(15)60692-4. - DOI - PMC - PubMed
-
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018; 38(1):1–211. 10.1177/0333102417738202. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

