The effect of Saccharomyces boulardii supplementation on Helicobacter pylori eradication in children: a systematic review and meta-analysis of Randomized controlled trials
- PMID: 38102568
- PMCID: PMC10722661
- DOI: 10.1186/s12879-023-08896-4
The effect of Saccharomyces boulardii supplementation on Helicobacter pylori eradication in children: a systematic review and meta-analysis of Randomized controlled trials
Abstract
Background: It is unclear whether Saccharomyces boulardii (S. boulardii) supplementation in standard triple therapy (STT) is effective in eradicating Helicobacter pylori (H. pylori) infection in children. We therefore conducted a meta-analysis of randomized controlled trials (RCTs) to assess the effect of S. boulardii supplementation on H. pylori eradication in children.
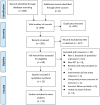
Methods: We conducted electronic searches in PubMed, Embase, the Cochrane Library, China National Knowledge Infrastructure and Wanfang database from the beginning up to September 2023. A random-effects model was employed to calculate the pooled relative risk (RR) with 95% confidence intervals (CI) through a meta-analysis.
Results: Fifteen RCTs (involving 2156 patients) were included in our meta-analysis. Results of the meta-analysis indicated that S. boulardii in combination with STT was more effective than STT alone (intention-to-treat analysis : 87.7% vs. 75.9%, RR = 1.14, 95% CI: 1.10-1.19, P < 0.00001; per-protocol analysis : 88.5% vs. 76.3%, RR = 1.15, 95% CI: 1.10-1.19, P < 0.00001). The S. boulardii supplementation group had a significantly lower incidence of total adverse events (n = 6 RCTs, 9.2% vs. 29.2%, RR = 0.32, 95% CI: 0.21-0.48, P < 0.00001), diarrhea (n = 13 RCTs, 14.7% vs. 32.4%, RR = 0.46, 95% CI: 0.37-0.56, P < 0.00001), and nausea (n = 11 RCTs, 12.7% vs. 21.3%, RR = 0.53, 95% CI: 0.40-0.72, P < 0.0001) than STT group alone. Similar results were also observed in the incidence of vomiting, constipation, abdominal pain, abdominal distention, epigastric discomfort, poor appetite and stomatitis.
Conclusions: Current evidence indicated that S. boulardii supplementing with STT could improve the eradication rate of H. pylori, and concurrently decrease the incidence of total adverse events and gastrointestinal adverse events in children.
Keywords: Helicobacter pylori; Meta-analysis; Probiotics; Randomized controlled trials; Saccharomyces boulardii; Systematic review.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
Effect of Saccharomyces boulardii supplementation to bismuth quadruple therapy on Helicobacter pylori eradication.BMC Gastroenterol. 2025 Apr 18;25(1):273. doi: 10.1186/s12876-025-03879-y. BMC Gastroenterol. 2025. PMID: 40251486 Free PMC article.
-
The effect of supplementing with Saccharomyces boulardii on bismuth quadruple therapy for eradicating Helicobacter pylori: a systematic review and meta-analysis of randomized controlled trials.Front Med (Lausanne). 2024 Apr 17;11:1344702. doi: 10.3389/fmed.2024.1344702. eCollection 2024. Front Med (Lausanne). 2024. PMID: 38695028 Free PMC article.
-
Efficacy and safety of Saccharomyces boulardii as an adjuvant therapy for the eradication of Helicobacter pylori: a meta-analysis.Front Cell Infect Microbiol. 2025 Feb 12;15:1441185. doi: 10.3389/fcimb.2025.1441185. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40012609 Free PMC article.
-
Saccharomyces boulardii as an adjuvant therapy for Helicobacter pylori eradication: A systematic review and meta-analysis with trial sequential analysis.Helicobacter. 2019 Oct;24(5):e12651. doi: 10.1111/hel.12651. Epub 2019 Aug 14. Helicobacter. 2019. PMID: 31414551
-
Efficacy of Probiotic Supplementation Therapy for Helicobacter pylori Eradication: A Meta-Analysis of Randomized Controlled Trials.PLoS One. 2016 Oct 10;11(10):e0163743. doi: 10.1371/journal.pone.0163743. eCollection 2016. PLoS One. 2016. PMID: 27723762 Free PMC article. Review.
Cited by
-
Efficacy of probiotics pretreatment in Helicobacter pylori eradication therapy: a systematic review and meta-analysis of clinical outcomes.Ann Med. 2025 Dec;57(1):2533431. doi: 10.1080/07853890.2025.2533431. Epub 2025 Jul 23. Ann Med. 2025. PMID: 40697099 Free PMC article.
-
Probiotics and prebiotics: new treatment strategies for oral potentially malignant disorders and gastrointestinal precancerous lesions.NPJ Biofilms Microbiomes. 2025 Apr 8;11(1):55. doi: 10.1038/s41522-025-00688-9. NPJ Biofilms Microbiomes. 2025. PMID: 40199865 Free PMC article. Review.
-
Effect of Saccharomyces boulardii on Liver Diseases: A Systematic Review.Microorganisms. 2024 Aug 15;12(8):1678. doi: 10.3390/microorganisms12081678. Microorganisms. 2024. PMID: 39203520 Free PMC article. Review.
-
A Comprehensive Review of the Diversity of Fungal Secondary Metabolites and Their Emerging Applications in Healthcare and Environment.Mycobiology. 2024 Dec 3;52(6):335-387. doi: 10.1080/12298093.2024.2416736. eCollection 2024. Mycobiology. 2024. PMID: 39845176 Free PMC article. Review.
References
-
- Malfertheiner P, Megraud F, Rokkas T, Gisbert JP, Liou JM, Schulz C et al. Management of Helicobacter pylori Infection: the Maastricht VI/Florence consensus report. Gut. 2022 Aug 8:gutjnl-2022-327745. 10.1136/gutjnl-2022-327745. Epub ahead of print.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

