A cross-sectional study on stool- and gastrointestinal-related outcomes of Mexican infants consuming different formulae
- PMID: 38102583
- PMCID: PMC10722798
- DOI: 10.1186/s12887-023-04426-y
A cross-sectional study on stool- and gastrointestinal-related outcomes of Mexican infants consuming different formulae
Abstract
Background: Immaturities present at birth, such as in the gut microbiome and digestive, nervous, and immune system, resolve with time. Nevertheless, this may result in mild digestive symptoms early in life, particularly in formula-fed infants. Formula composition and processing may impact this discomfort. This study therefore aimed to assess stool characteristics and gastrointestinal symptoms of healthy infants fed different formulae.
Methods: A multicenter, cross-sectional, observational trial was performed in Mexico between November 2019 and January 2022, where exclusively formula-fed infants (n = 342, aged 1-4 months) were studied in four groups based on their existing formula use. Feeding was continued per practice following label instructions. For 7 days, parents/caregivers were requested to record fecal characteristics, using the Amsterdam Infant Stool Scale, and rate gastrointestinal symptoms. Stool samples were collected to determine pH, dry matter content, and fecal calprotectin levels.
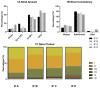
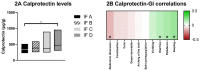
Results: Most infants had a soft/formed stool consistency, although odds for hard stools were different between groups. Gastrointestinal symptom scores revealed significant differences for burping and diarrhea, while other symptoms did not differ between groups. No significant differences between groups were found for stool frequency, dry matter content, and fecal pH. Although calprotectin was within the expected healthy ranges, significant differences among groups were seen. Furthermore, calprotectin significantly correlated with the severity of the gastrointestinal symptoms burping, flatulence, abdominal distension, and diarrhea.
Conclusions: Differences in stool characteristics and specific differences in gastrointestinal symptoms were observed between different formula brand users. This may potentially be explained by the different composition and processing of the formulae, although there are multiple factors that influence the assessed outcomes.
Trial registration: The study was registered in the Netherlands Trial Registry (NL7805), linked to https://trialsearch.who.int/ , on 11/06/2019.
Keywords: Digestive symptoms; Fecal characteristics; Infant formula; Infant nutrition.
© 2023. The Author(s).
Conflict of interest statement
CMM, JHJH, MCF, and TTL are employed by FrieslandCampina. For the remaining authors, none is declared. This study was funded by FrieslandCampina, Amersfoort, The Netherlands.
Figures
Similar articles
-
Effects of term infant formulas containing high sn-2 palmitate with and without oligofructose on stool composition, stool characteristics, and bifidogenicity.J Pediatr Gastroenterol Nutr. 2014 Oct;59(4):440-8. doi: 10.1097/MPG.0000000000000443. J Pediatr Gastroenterol Nutr. 2014. PMID: 24840511 Free PMC article. Clinical Trial.
-
Stool fatty acid soaps, stool consistency and gastrointestinal tolerance in term infants fed infant formulas containing high sn-2 palmitate with or without oligofructose: a double-blind, randomized clinical trial.Nutr J. 2014 Nov 5;13:105. doi: 10.1186/1475-2891-13-105. Nutr J. 2014. PMID: 25373935 Free PMC article. Clinical Trial.
-
High faecal calprotectin levels in healthy, exclusively breast-fed infants.Neonatology. 2010 Jun;97(4):299-304. doi: 10.1159/000255161. Epub 2009 Nov 4. Neonatology. 2010. PMID: 19887860 Clinical Trial.
-
Importance of the regiospecific distribution of long-chain saturated fatty acids on gut comfort, fat and calcium absorption in infants.Prostaglandins Leukot Essent Fatty Acids. 2017 Jun;121:40-51. doi: 10.1016/j.plefa.2017.05.007. Epub 2017 Jun 4. Prostaglandins Leukot Essent Fatty Acids. 2017. PMID: 28651696 Review.
-
Safety and efficacy of adding postbiotics in infant formula: a systematic review and meta-analysis.Pediatr Res. 2024 Jan;95(1):43-51. doi: 10.1038/s41390-023-02813-w. Epub 2023 Sep 12. Pediatr Res. 2024. PMID: 37700163
References
-
- Lindquist S, Hernell O. Lipid digestion and absorption in early life: an update. Curr Opin Clin Nutr Metab Care [Internet]. 2010 [cited 2022 Nov 28];13(3):314–20. Available from: https://pubmed.ncbi.nlm.nih.gov/20179589/. - PubMed
-
- Bourlieu C, Ménard O, Bouzerzour K, Mandalari G, Macierzanka A, Mackie AR et al. Specificity of infant digestive conditions: some clues for developing relevant in vitro models. Crit Rev Food Sci Nutr [Internet]. 2014 [cited 2022 Jan 6];54(11):1427–57. Available from: https://pubmed.ncbi.nlm.nih.gov/24580539/. - PubMed
-
- Indrio F, Neu J, Pettoello-Mantovani M, Marchese F, Martini S, Salatto A et al. Development of the Gastrointestinal Tract in Newborns as a Challenge for an Appropriate Nutrition: A Narrative Review. Nutrients [Internet]. 2022 Apr 1 [cited 2023 Aug 25];14(7). Available from: https://pubmed.ncbi.nlm.nih.gov/35406018/. - PMC - PubMed
-
- Pina DI, Llach XB, Ariño-Armengol B, Iglesias VV. Prevalence and dietetic management of mild gastrointestinal disorders in milk-fed infants. World J Gastroenterol [Internet]. 2008 Jan 14 [cited 2023 Jan 16];14(2):248–54. Available from: https://pubmed.ncbi.nlm.nih.gov/18186563/. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

