Neuron-targeted overexpression of caveolin-1 alleviates diabetes-associated cognitive dysfunction via regulating mitochondrial fission-mitophagy axis
- PMID: 38102662
- PMCID: PMC10722701
- DOI: 10.1186/s12964-023-01328-5
Neuron-targeted overexpression of caveolin-1 alleviates diabetes-associated cognitive dysfunction via regulating mitochondrial fission-mitophagy axis
Abstract
Background: Type 2 diabetes mellitus (T2DM) induced diabetes-associated cognitive dysfunction (DACD) that seriously affects the self-management of T2DM patients, is currently one of the most severe T2DM-associated complications, but the mechanistic basis remains unclear. Mitochondria are highly dynamic organelles, whose function refers to a broad spectrum of features such as mitochondrial dynamics, mitophagy and so on. Mitochondrial abnormalities have emerged as key determinants for cognitive function, the relationship between DACD and mitochondria is not well understood.
Methods: Here, we explored the underlying mechanism of mitochondrial dysfunction of T2DM mice and HT22 cells treated with high glucose/palmitic acid (HG/Pal) focusing on the mitochondrial fission-mitophagy axis with drug injection, western blotting, Immunofluorescence, and electron microscopy. We further explored the potential role of caveolin-1 (cav-1) in T2DM induced mitochondrial dysfunction and synaptic alteration through viral transduction.
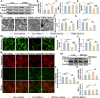
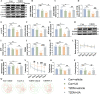
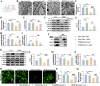
Results: As previously reported, T2DM condition significantly prompted hippocampal mitochondrial fission, whereas mitophagy was blocked rather than increasing, which was accompanied by dysfunctional mitochondria and impaired neuronal function. By contrast, Mdivi-1 (mitochondrial division inhibitor) and urolithin A (mitophagy activator) ameliorated mitochondrial and neuronal function and thereafter lead to cognitive improvement by inhibiting excessive mitochondrial fission and giving rise to mitophagy, respectively. We have previously shown that cav-1 can significantly improve DACD by inhibiting ferroptosis. Here, we further demonstrated that cav-1 could not only inhibit mitochondrial fission via the interaction with GSK3β to modulate Drp1 pathway, but also rescue mitophagy through interacting with AMPK to activate PINK1/Parkin and ULK1-dependent signlings.
Conclusions: Overall, our data for the first time point to a mitochondrial fission-mitophagy axis as a driver of neuronal dysfunction in a phenotype that was exaggerated by T2DM, and the protective role of cav-1 in DACD. Graphic Summary Illustration. In T2DM, excessive mitochondrial fission and impaired mitophagy conspire to an altered mitochondrial morphology and mitochondrial dysfunction, with a consequent neuronal damage, overall suggesting an unbalanced mitochondrial fission-mitophagy axis. Upon cav-1 overexpression, GSK3β and AMPK are phosphorylated respectively to activate Drp1 and mitophagy-related pathways (PINK1 and ULKI), ultimately inhibits mitochondrial fission and enhances mitophagy. In the meantime, the mitochondrial morphology and neuronal function are rescued, indicating the protective role of cav-1 on mitochondrial fission-mitophagy axis. Video Abstract.
Keywords: Caveolin-1; Diabetes-associated cognitive dysfunction; Mitochondrial fission; Mitophagy.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- van Gennip ACE, Stehouwer CDA, van Boxtel MPJ, Verhey FRJ, Koster A, Kroon AA, et al. Association of Type 2 Diabetes, According to the Number of Risk Factors Within Target Range, With Structural Brain Abnormalities, Cognitive Performance, and Risk of Dementia. Diabetes Care. 2021;44(11):2493–2502. doi: 10.2337/dc21-0149. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 2023-JC-QN-0928/Natural Science Basic Research Program of Shanxi
- 2022JQ-819/Natural Science Basic Research Program of Shanxi
- 81801899/National Natural Science Foundation of China
- 81974540/National Natural Science Foundation of China
- 2022ZDLSF02-09/the Key Research & Development Program of Shanxi
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

