RGCC-mediated PLK1 activity drives breast cancer lung metastasis by phosphorylating AMPKα2 to activate oxidative phosphorylation and fatty acid oxidation
- PMID: 38102722
- PMCID: PMC10722681
- DOI: 10.1186/s13046-023-02928-2
RGCC-mediated PLK1 activity drives breast cancer lung metastasis by phosphorylating AMPKα2 to activate oxidative phosphorylation and fatty acid oxidation
Abstract
Background: More than 90% of the mortality of triple-negative breast cancer (TNBC) patients is attributed to cancer metastasis with organotropism. The lung is a frequent site of TNBC metastasis. However, the precise molecular mechanism for lung-specific metastasis of TNBC is not well understood.
Methods: RNA sequencing was performed to identify patterns of gene expression associated with lung metastatic behavior using 4T1-LM3, MBA-MB-231-LM3, and their parental cells (4T1-P, MBA-MB-231-P). Expressions of RGCC, called regulator of cell cycle or response gene to complement 32 protein, were detected in TNBC cells and tissues by qRT-PCR, western blotting, and immunohistochemistry. Kinase activity assay was performed to evaluate PLK1 kinase activity. The amount of phosphorylated AMP-activated protein kinase α2 (AMPKα2) was detected by immunoblotting. RGCC-mediated metabolism was determined by UHPLC system. Oxidative phosphorylation was evaluated by JC-1 staining and oxygen consumption rate (OCR) assay. Fatty acid oxidation assay was conducted to measure the status of RGCC-mediated fatty acid oxidation. NADPH and ROS levels were detected by well-established assays. The chemical sensitivity of cells was evaluated by CCK8 assay.
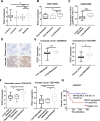
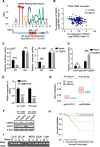
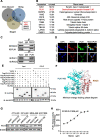
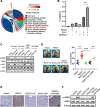
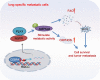
Results: RGCC is aberrantly upregulated in pulmonary metastatic cells. High level of RGCC is significantly related with lung metastasis in comparison with other organ metastases. RGCC can effectively promote kinase activity of PLK1, and the activated PLK1 phosphorylates AMPKα2 to facilitate TNBC lung metastasis. Mechanistically, the RGCC/PLK1/AMPKα2 signal axis increases oxidative phosphorylation of mitochondria to generate more energy, and promotes fatty acid oxidation to produce abundant NADPH. These metabolic changes contribute to sustaining redox homeostasis and preventing excessive accumulation of potentially detrimental ROS in metastatic tumor cells, thereby supporting TNBC cell survival and colonization during metastases. Importantly, targeting RGCC in combination with paclitaxel/carboplatin effectively suppresses pulmonary TNBC lung metastasis in a mouse model.
Conclusions: RGCC overexpression is significantly associated with lung-specific metastasis of TNBC. RGCC activates AMPKα2 and downstream signaling through RGCC-driven PLK1 activity to facilitate TNBC lung metastasis. The study provides implications for RGCC-driven OXPHOS and fatty acid oxidation as important therapeutic targets for TNBC treatment.
Keywords: Fatty acid oxidation; Lung metastasis; OXPHOS; PLK1; RGCC.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Garrido-Castro AC, Lin NU, Polyak K. Insights into molecular classifications of triple-negative breast cancer: improving patient selection for treatment. Cancer Discov. 2019;9(2):176–198. doi: 10.1158/2159-8290.CD-18-1177. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

