Extracellular Vesicles Derived From 3D Cultured Antler Stem Cells Serve as a New Drug Vehicle in Osteosarcoma Treatment
- PMID: 38102784
- PMCID: PMC10725652
- DOI: 10.1177/09636897231219830
Extracellular Vesicles Derived From 3D Cultured Antler Stem Cells Serve as a New Drug Vehicle in Osteosarcoma Treatment
Abstract
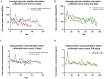
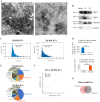
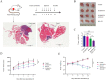
Extracellular vesicles (EVs) from antler reserve mesenchymal (RM) cells play an important role in the paracrine regulation during rapid growth of antler without forming a tumor; therefore, RM-EVs become novel materials for anti-tumor studies, such as osteosarcoma treatment. However, the problem of low production of RM-EVs in traditional 2D culture limits its mechanism research and application. In this study, we established an optimal 3D culture system for antler RM cells to produce EVs (3D-RM-EVs). Morphology and property of harvested 3D-RM-EVs were normal compared with EVs from conventional 2D culture, and the miRNA profile in them was basically the same through transcriptome sequencing analysis. Based on the same number of RM cells, the volume of the culture medium collected by 3D cultural system concentrated nearly 30 times, making it more convenient for subsequent purification. In addition, EVs were harvested 30 times in 3D cultural system, greatly increasing the total amount of EVs (harvested a total of 2-3 times in 2D culture). Although 3D-RM-EVs had a limited inhibitory effect on the proliferation of K7M2 cells, the inhibition effect of 3D-RM-EVs loaded drugs (Ifosfamide + Etoposide) were more significant than that of positive drug group alone (P < 0.05). Furthermore, in vivo studies showed that 3D-RM-EVs loaded drugs (Ifosfamide + Etoposide) had the most significant tumor inhibition effect, with decreased tumor size, and could slow down body weight loss compared with Ifosfamide + Etoposide (IFO + ET) group. These results demonstrated that 3D-RM-EVs were efficiently prepared from antler RM cells and were effective as drug vehicles for the treatment of osteosarcoma.
Keywords: 3D cell culture; antler reserve mesenchymal cell; extracellular vesicles; hollow fiber cell culture; osteosarcoma.
Conflict of interest statement
Declaration of Conflicting InterestsThe author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures


Similar articles
-
PRRX1/miR-143-3p signaling regulates homeostasis of antler reserve mesenchymal cells.Int J Biol Macromol. 2025 Jan;285:138366. doi: 10.1016/j.ijbiomac.2024.138366. Epub 2024 Dec 5. Int J Biol Macromol. 2025. PMID: 39638193
-
Bone marrow mesenchymal stromal cells in a 3D system produce higher concentration of extracellular vesicles (EVs) with increased complexity and enhanced neuronal growth properties.Stem Cell Res Ther. 2022 Aug 19;13(1):425. doi: 10.1186/s13287-022-03128-z. Stem Cell Res Ther. 2022. PMID: 35986305 Free PMC article.
-
A 3D culture system improves the yield of MSCs-derived extracellular vesicles and enhances their therapeutic efficacy for heart repair.Biomed Pharmacother. 2023 May;161:114557. doi: 10.1016/j.biopha.2023.114557. Epub 2023 Mar 22. Biomed Pharmacother. 2023. PMID: 36963364
-
The role of chemotherapy for metastatic, relapsed and refractory osteosarcoma.Paediatr Drugs. 2014 Dec;16(6):503-12. doi: 10.1007/s40272-014-0095-z. Paediatr Drugs. 2014. PMID: 25392156 Review.
-
Advances in the application of extracellular vesicles derived from three-dimensional culture of stem cells.J Nanobiotechnology. 2024 May 1;22(1):215. doi: 10.1186/s12951-024-02455-y. J Nanobiotechnology. 2024. PMID: 38693585 Free PMC article. Review.
References
-
- Kourembanas S. Exosomes: vehicles of intercellular signaling, biomarkers, and vectors of cell therapy. Annu Rev Physiol. 2015;77:13–27. - PubMed
-
- Hassanzadeh A, Rahman HS, Markov A, Endjun JJ, Zekiy AO, Chartrand MS, Beheshtkhoo N, Kouhbanani M, Marofi F, Nikoo M, Jarahian M. Mesenchymal stem/stromal cell-derived exosomes in regenerative medicine and cancer; overview of development, challenges, and opportunities. Stem Cell Res Ther. 2021;12(1):297. - PMC - PubMed
-
- Chen BY, Sung CW, Chen C, Cheng CM, Lin DP, Huang CT, Hsu MY. Advances in exosomes technology. Clin Chim Acta. 2019;493:14–19. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

