Sprouting of afferent and efferent inputs to pelvic organs after spinal cord injury
- PMID: 38102789
- PMCID: PMC10746698
- DOI: 10.1093/jnen/nlad108
Sprouting of afferent and efferent inputs to pelvic organs after spinal cord injury
Abstract
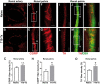
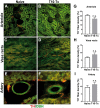
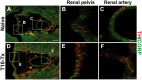
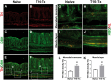
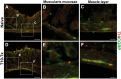
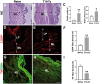
Neural plasticity occurs within the central and peripheral nervous systems after spinal cord injury (SCI). Although central alterations have extensively been studied, it is largely unknown whether afferent and efferent fibers in pelvic viscera undergo similar morphological changes. Using a rat spinal cord transection model, we conducted immunohistochemistry to investigate afferent and efferent innervations to the kidney, colon, and bladder. Approximately 3-4 weeks after injury, immunostaining demonstrated that tyrosine hydroxylase (TH)-labeled postganglionic sympathetic fibers and calcitonin gene-related peptide (CGRP)-immunoreactive sensory terminals sprout in the renal pelvis and colon. Morphologically, sprouted afferent or efferent projections showed a disorganized structure. In the bladder, however, denser CGRP-positive primary sensory fibers emerged in rats with SCI, whereas TH-positive sympathetic efferent fibers did not change. Numerous CGRP-positive afferents were observed in the muscle layer and the lamina propria of the bladder following SCI. TH-positive efferent inputs displayed hypertrophy with large diameters, but their innervation patterns were sustained. Collectively, afferent or efferent inputs sprout widely in the pelvic organs after SCI, which may be one of the morphological bases underlying functional adaptation or maladaptation.
Keywords: Calcitonin gene-related peptide; Distribution; Peripheral system; Sprout; Tyrosine hydroxylase.
© The Author(s) 2023. Published by Oxford University Press on behalf of American Association of Neuropathologists, Inc. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Conflict of interest statement
The authors have no duality or conflicts of interest to declare.
Figures

Similar articles
-
Intraspinal sprouting of unmyelinated pelvic afferents after complete spinal cord injury is correlated with autonomic dysreflexia induced by visceral pain.Neuroscience. 2009 Mar 3;159(1):369-79. doi: 10.1016/j.neuroscience.2008.12.022. Epub 2008 Dec 24. Neuroscience. 2009. PMID: 19146928 Free PMC article.
-
Sprouting of primary afferent fibers after spinal cord transection in the rat.Neuroscience. 1998 Jul;85(2):443-58. doi: 10.1016/s0306-4522(97)00622-2. Neuroscience. 1998. PMID: 9622243
-
Reconstruction of atonic bladder innervation after spinal cord injury: A bladder reflex arc with afferent and efferent pathways.J Spinal Cord Med. 2015 Nov;38(6):717-28. doi: 10.1179/2045772314Y.0000000285. Epub 2015 Jan 13. J Spinal Cord Med. 2015. PMID: 25582052 Free PMC article.
-
Segmental organization of spinal reflexes mediating autonomic dysreflexia after spinal cord injury.Prog Brain Res. 2006;152:265-74. doi: 10.1016/S0079-6123(05)52017-X. Prog Brain Res. 2006. PMID: 16198706 Free PMC article. Review.
-
Neuropeptides in pelvic afferent pathways.Experientia. 1987 Jul 15;43(7):801-13. doi: 10.1007/BF01945358. Experientia. 1987. PMID: 3297768 Review.
Cited by
-
Renal interoception: form, function, and open questions.Curr Opin Neurobiol. 2025 Aug;93:103067. doi: 10.1016/j.conb.2025.103067. Epub 2025 Jun 25. Curr Opin Neurobiol. 2025. PMID: 40570502 Review.
References
-
- Rank MM, Flynn JR, Galea MP, et al.Electrophysiological characterization of spontaneous recovery in deep dorsal horn interneurons after incomplete spinal cord injury. Exp Neurol 2015;271:468–78 - PubMed
-
- Blesch A, Tuszynski MH.. Spinal cord injury: Plasticity, regeneration and the challenge of translational drug development. Trends Neurosci 2009;32:41–7 - PubMed
-
- Bareyre FM, Kerschensteiner M, Raineteau O, et al.The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci 2004;7:269–77 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

