Hyperexcitability of muscle spindle afferents in jaw-closing muscles in experimental myalgia: Evidence for large primary afferents involvement in chronic pain
- PMID: 38103003
- PMCID: PMC10988680
- DOI: 10.1113/EP090769
Hyperexcitability of muscle spindle afferents in jaw-closing muscles in experimental myalgia: Evidence for large primary afferents involvement in chronic pain
Abstract
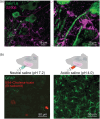
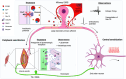
The goals of this review are to improve understanding of the aetiology of chronic muscle pain and identify new targets for treatments. Muscle pain is usually associated with trigger points in syndromes such as fibromyalgia and myofascial syndrome, and with small spots associated with spontaneous electrical activity that seems to emanate from fibers inside muscle spindles in EMG studies. These observations, added to the reports that large-diameter primary afferents, such as those innervating muscle spindles, become hyperexcitable and develop spontaneous ectopic firing in conditions leading to neuropathic pain, suggest that changes in excitability of these afferents might make an important contribution to the development of pathological pain. Here, we review evidence that the muscle spindle afferents (MSAs) of the jaw-closing muscles become hyperexcitable in a model of chronic orofacial myalgia. In these afferents, as in other large-diameter primary afferents in dorsal root ganglia, firing emerges from fast membrane potential oscillations that are supported by a persistent sodium current (INaP ) mediated by Na+ channels containing the α-subunit NaV 1.6. The current flowing through NaV 1.6 channels increases when the extracellular Ca2+ concentration decreases, and studies have shown that INaP -driven firing is increased by S100β, an astrocytic protein that chelates Ca2+ when released in the extracellular space. We review evidence of how astrocytes, which are known to be activated in pain conditions, might, through their regulation of extracellular Ca2+ , contribute to the generation of ectopic firing in MSAs. To explain how ectopic firing in MSAs might cause pain, we review evidence supporting the hypothesis that cross-talk between proprioceptive and nociceptive pathways might occur in the periphery, within the spindle capsule.
Keywords: astrocytes; chronic muscle pain; ectopic firing; muscle spindle afferent; trigeminal system.
© 2023 The Authors. Experimental Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Conflict of interest statement
None declared.
Figures


References
-
- Abe, M. , Kurihara, T. , Han, W. , Shinomiya, K. , & Tanabe, T. (2002). Changes in expression of voltage‐dependent ion channel subunits in dorsal root ganglia of rats with radicular injury and pain. Spine (Phila Pa 1976), 27(14), 1517–1524. discussion 1525. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
