Angiotensin II treatment is associated with improved oxygenation in ARDS patients with refractory vasodilatory shock
- PMID: 38103056
- PMCID: PMC10725390
- DOI: 10.1186/s13613-023-01227-5
Angiotensin II treatment is associated with improved oxygenation in ARDS patients with refractory vasodilatory shock
Abstract
Background: The physiological effects of renin-angiotensin system modulation in acute respiratory distress syndrome (ARDS) remain controversial and have not been investigated in randomized trials. We sought to determine whether angiotensin-II treatment is associated with improved oxygenation in shock-associated ARDS.
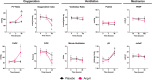
Methods: Post-hoc subgroup analysis of the Angiotensin Therapy for High Output Shock (ATHOS-3) trial. We studied patients who met modified Berlin ARDS criteria at enrollment. The primary outcome was PaO2/FiO2-ratio (P:F) at 48-h adjusted for baseline P:F. Secondary outcomes included oxygenation index, ventilatory ratio, PEEP, minute-ventilation, hemodynamic measures, patients alive and ventilator-free by day-7, and mortality.
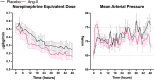
Results: Of 81 ARDS patients, 34 (42%) and 47 (58%) were randomized to angiotensin-II or placebo, respectively. In angiotensin-II patients, mean P:F increased from 155 mmHg (SD: 69) at baseline to 265 mmHg (SD: 160) at hour-48 compared with no change with placebo (148 mmHg (SD: 63) at baseline versus 164 mmHg (SD: 74) at hour-48)(baseline-adjusted difference: + 98.4 mmHg [95%CI 35.2-161.5], p = 0.0028). Similarly, oxygenation index decreased by - 6.0 cmH2O/mmHg at hour-48 with angiotensin-II versus - 0.4 cmH2O/mmHg with placebo (baseline-adjusted difference: -4.8 cmH2O/mmHg, [95%CI - 8.6 to - 1.1], p = 0.0273). There was no difference in PEEP, minute ventilation, or ventilatory ratio. Twenty-two (64.7%) angiotensin-II patients had sustained hemodynamic response to treatment at hour-3 versus 17 (36.2%) placebo patients (absolute risk-difference: 28.5% [95%CI 6.5-47.0%], p = 0.0120). At day-7, 7/34 (20.6%) angiotensin-II patients were alive and ventilator-free versus 5/47(10.6%) placebo patients. Day-28 mortality was 55.9% in the angiotensin-II group versus 68.1% in the placebo group.
Conclusions: In post-hoc analysis of the ATHOS-3 trial, angiotensin-II was associated with improved oxygenation versus placebo among patients with ARDS and catecholamine-refractory vasodilatory shock. These findings provide a physiologic rationale for trials of angiotensin-II as treatment for ARDS with vasodilatory shock.
Trial registration: ClinicalTrials.Gov Identifier: NCT02338843 (Registered January 14th 2015).
Keywords: ARDS; Angiotensin II; Norepinephrine; Renin–angiotensin system; Septic; Shock.
© 2023. The Author(s).
Conflict of interest statement
DEL, AMD, KRH, and RB declare no competing interests. DRH, CDA, and TNH are employees of Innoviva Specialty Therapeutics, of which La Jolla Pharmaceutical Company (LJPC) is a subsidiary. LSC declares that he was formerly an employee of LJPC. TEA received consulting fees from LJPC. LWB served on the Speaker’s Bureau for LJPC and received consulting fees from LJPC. DWB served on the Speaker’s Bureau for LJPC and received consulting fees from LJPC. MNG received NIH and CDC grants for research unrelated to this study, fees for serving on scientific advisory panel for Philips Healthcare and Endpoint for advice on AI and personalized approach to sepsis, and previously received funding from LJPC for the conduct of the Athos-3 trial. AKK served on the Speaker’s Bureau for LJPC, received consulting fees from LJPC, and received research grant funding from La Jolla Pharmaceutical Company for the ATHOS-3 study and through the Wake Forest Center for Hypertension and Vascular Research for RAAS in septic shock. MTM served on the Speaker’s Bureau for LJPC. MO declares that her institution received research funding from LJPC. BTT declares that during a portion of this research, the author had a financial interest in Direct Biologics, a developer and manufacturer of regenerative biologic products, including an investigational treatment of COVID-19 associated ARDS. These interests were reviewed and are managed by Massachusetts General Hospital and Mass General Brigham in accordance with their conflict of interest policies. JTT received research grant support and institutional funding from LJPC.
Figures

References
-
- Lawler PR, Derde LPG, van de Veerdonk FL, et al. Effect of angiotensin-converting enzyme inhibitor and angiotensin receptor blocker initiation on organ support-free days in patients hospitalized with COVID-19: a randomized clinical trial. JAMA. 2023;329(14):1183–1196. doi: 10.1001/jama.2023.4480. - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

