Analysis of biomarkers in speculative CNS-enriched extracellular vesicles for parkinsonian disorders: a comprehensive systematic review and diagnostic meta-analysis
- PMID: 38103086
- PMCID: PMC10973014
- DOI: 10.1007/s00415-023-12093-3
Analysis of biomarkers in speculative CNS-enriched extracellular vesicles for parkinsonian disorders: a comprehensive systematic review and diagnostic meta-analysis
Abstract
Background and objective: Parkinsonian disorders, including Parkinson's disease (PD), multiple system atrophy (MSA), dementia with Lewy bodies (DLB), progressive supranuclear palsy (PSP), and corticobasal syndrome (CBS), exhibit overlapping early-stage symptoms, complicating definitive diagnosis despite heterogeneous cellular and regional pathophysiology. Additionally, the progression and the eventual conversion of prodromal conditions such as REM behavior disorder (RBD) to PD, MSA, or DLB remain challenging to predict. Extracellular vesicles (EVs) are small, membrane-enclosed structures released by cells, playing a vital role in communicating cell-state-specific messages. Due to their ability to cross the blood-brain barrier into the peripheral circulation, measuring biomarkers in blood-isolated speculative CNS enriched EVs has become a popular diagnostic approach. However, replication and independent validation remain challenging in this field. Here, we aimed to evaluate the diagnostic accuracy of speculative CNS-enriched EVs for parkinsonian disorders.
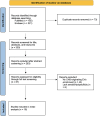
Methods: We conducted a PRISMA-guided systematic review and meta-analysis, covering 18 studies with a total of 1695 patients with PD, 253 with MSA, 21 with DLB, 172 with PSP, 152 with CBS, 189 with RBD, and 1288 HCs, employing either hierarchical bivariate models or univariate models based on study size.
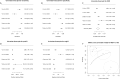
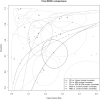
Results: Diagnostic accuracy was moderate for differentiating patients with PD from HCs, but revealed high heterogeneity and significant publication bias, suggesting an inflation of the perceived diagnostic effectiveness. The bias observed indicates that studies with non-significant or lower effect sizes were less likely to be published. Although results for differentiating patients with PD from those with MSA or PSP and CBS appeared promising, their validity is limited due to the small number of involved studies coming from the same research group. Despite initial reports, our analyses suggest that using speculative CNS-enriched EV biomarkers may not reliably differentiate patients with MSA from HCs or patients with RBD from HCs, due to their lesser accuracy and substantial variability among the studies, further complicated by substantial publication bias.
Conclusion: Our findings underscore the moderate, yet unreliable diagnostic accuracy of biomarkers in speculative CNS-enriched EVs in differentiating parkinsonian disorders, highlighting the presence of substantial heterogeneity and significant publication bias. These observations reinforce the need for larger, more standardized, and unbiased studies to validate the utility of these biomarkers but also call for the development of better biomarkers for parkinsonian disorders.
Keywords: Alpha-synuclein; Diagnosis; Exosome; L1CAM; Movement disorders; Parkinson’s disease.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






References
-
- Blommer J, Pitcher T, Mustapic M, Eren E, Yao PJ, Vreones MP, Pucha KA, Dalrymple-Alford J, Shoorangiz R, Meissner WG, Anderson T, Kapogiannis D. Extracellular vesicle biomarkers for cognitive impairment in Parkinson's disease. Brain. 2023;146:195–208. doi: 10.1093/brain/awac258. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

