Examining memory linking and generalization using scFLARE2, a temporally precise neuronal activity tagging system
- PMID: 38103203
- PMCID: PMC10842737
- DOI: 10.1016/j.celrep.2023.113592
Examining memory linking and generalization using scFLARE2, a temporally precise neuronal activity tagging system
Abstract
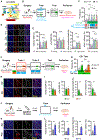
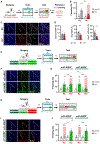
How memories are organized in the brain influences whether they are remembered discretely versus linked with other experiences or whether generalized information is applied to entirely novel situations. Here, we used scFLARE2 (single-chain fast light- and activity-regulated expression 2), a temporally precise tagging system, to manipulate mouse lateral amygdala neurons active during one of two 3 min threat experiences occurring close (3 h) or further apart (27 h) in time. Silencing scFLARE2-tagged neurons showed that two threat experiences occurring at distal times are dis-allocated to orthogonal engram ensembles and remembered discretely, whereas the same two threat experiences occurring in close temporal proximity are linked via co-allocation to overlapping engram ensembles. Moreover, we found that co-allocation mediates memory generalization applied to a completely novel stimulus. These results indicate that endogenous temporal evolution of engram ensemble neuronal excitability determines how memories are organized and remembered and that this would not be possible using conventional immediate-early gene-based tagging methods.
Keywords: CP: Neuroscience; amygdala; coding schemes; engram ensembles; memory; memory generalization; memory linking; mouse; neuronal-activity-dependent tagging systems; optogenetics; scFLARE2.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


References
-
- Josselyn SA, Köhler S, and Frankland PW (2015). Finding the engram. Nat. Rev. Neurosci 16, 521–534. - PubMed
-
- Tonegawa S, Pignatelli M, Roy DS, and Ryan TJ (2015). Memory engram storage and retrieval. Curr. Opin. Neurobiol 35, 101–109. - PubMed
-
- Han DH, Park P, Choi DI, Bliss TVP, and Kaang B-K (2022). The essence of the engram: Cellular or synaptic? Semin. Cell Dev. Biol 125, 122–135. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

