Lupenone improves motor dysfunction in spinal cord injury mice through inhibiting the inflammasome activation and pyroptosis in microglia via the nuclear factor kappa B pathway
- PMID: 38103247
- PMCID: PMC10960275
- DOI: 10.4103/1673-5374.389302
Lupenone improves motor dysfunction in spinal cord injury mice through inhibiting the inflammasome activation and pyroptosis in microglia via the nuclear factor kappa B pathway
Abstract
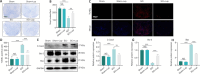
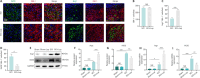
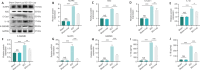
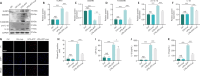
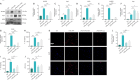
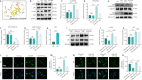
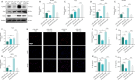
JOURNAL/nrgr/04.03/01300535-202408000-00034/figure1/v/2023-12-16T180322Z/r/image-tiff Spinal cord injury-induced motor dysfunction is associated with neuroinflammation. Studies have shown that the triterpenoid lupenone, a natural product found in various plants, has a remarkable anti-inflammatory effect in the context of chronic inflammation. However, the effects of lupenone on acute inflammation induced by spinal cord injury remain unknown. In this study, we established an impact-induced mouse model of spinal cord injury, and then treated the injured mice with lupenone (8 mg/kg, twice a day) by intraperitoneal injection. We also treated BV2 cells with lipopolysaccharide and adenosine 5'-triphosphate to simulate the inflammatory response after spinal cord injury. Our results showed that lupenone reduced IκBα activation and p65 nuclear translocation, inhibited NLRP3 inflammasome function by modulating nuclear factor kappa B, and enhanced the conversion of proinflammatory M1 microglial cells into anti-inflammatory M2 microglial cells. Furthermore, lupenone decreased NLRP3 inflammasome activation, NLRP3-induced microglial cell polarization, and microglia pyroptosis by inhibiting the nuclear factor kappa B pathway. These findings suggest that lupenone protects against spinal cord injury by inhibiting inflammasomes.
Copyright © 2024 Copyright: © 2024 Neural Regeneration Research.
Conflict of interest statement
Figures


Similar articles
-
Inhibiting ceramide synthase 5 expression in microglia decreases neuroinflammation after spinal cord injury.Neural Regen Res. 2025 Oct 1;20(10):2955-2968. doi: 10.4103/NRR.NRR-D-23-01933. Epub 2024 Jun 3. Neural Regen Res. 2025. PMID: 39610106 Free PMC article.
-
TLR4 aggravates microglial pyroptosis by promoting DDX3X-mediated NLRP3 inflammasome activation via JAK2/STAT1 pathway after spinal cord injury.Clin Transl Med. 2022 Jun;12(6):e894. doi: 10.1002/ctm2.894. Clin Transl Med. 2022. PMID: 35692100 Free PMC article.
-
Oral treatment with glycyrrhizin inhibits NLRP3 inflammasome activation and promotes microglial M2 polarization after traumatic spinal cord injury.Brain Res Bull. 2020 May;158:1-8. doi: 10.1016/j.brainresbull.2020.02.009. Epub 2020 Feb 21. Brain Res Bull. 2020. PMID: 32092434
-
Salvianolic Acids for Injection alleviates cerebral ischemia/reperfusion injury by switching M1/M2 phenotypes and inhibiting NLRP3 inflammasome/pyroptosis axis in microglia in vivo and in vitro.J Ethnopharmacol. 2021 Apr 24;270:113776. doi: 10.1016/j.jep.2021.113776. Epub 2021 Jan 7. J Ethnopharmacol. 2021. PMID: 33421597
-
Biochanin A attenuates spinal cord injury in rats during early stages by inhibiting oxidative stress and inflammasome activation.Neural Regen Res. 2024 Sep 1;19(9):2050-2056. doi: 10.4103/1673-5374.390953. Epub 2023 Dec 15. Neural Regen Res. 2024. PMID: 38227535 Free PMC article.
Cited by
-
CTRP9 attenuates peripheral nerve injury-induced mechanical allodynia and thermal hyperalgesia through regulating spinal microglial polarization and neuroinflammation mediated by AdipoR1 in male mice.Cell Biol Toxicol. 2024 Oct 26;40(1):91. doi: 10.1007/s10565-024-09933-x. Cell Biol Toxicol. 2024. PMID: 39460844 Free PMC article.
-
SP3-Mediated Transcriptional Activation of GRIK1 is Involved in Alzheimer's Disease-Induced Cognitive Decline by Inducing Inflammasome Activation in Microglia.Neuromolecular Med. 2025 Mar 7;27(1):22. doi: 10.1007/s12017-025-08844-2. Neuromolecular Med. 2025. PMID: 40055261
-
Role of MS4A7 in Regulating Microglial Polarization and Neuroinflammation in Spinal Cord Injury via the cGAS-STING-NLRP3 Axis.CNS Neurosci Ther. 2025 Jun;31(6):e70390. doi: 10.1111/cns.70390. CNS Neurosci Ther. 2025. PMID: 40522023 Free PMC article.
-
Antin-diabetic cognitive dysfunction effects and underpinning mechanisms of phytogenic bioactive peptides: a review.Front Nutr. 2025 Jan 10;11:1517087. doi: 10.3389/fnut.2024.1517087. eCollection 2024. Front Nutr. 2025. PMID: 39867560 Free PMC article. Review.
-
Limosilactobacillus reuteri DSM17938 Attenuates Neuroinflammatory Responses After Spinal Cord Injury by Modulating Tryptophan Metabolism.Probiotics Antimicrob Proteins. 2025 Apr 26. doi: 10.1007/s12602-025-10545-y. Online ahead of print. Probiotics Antimicrob Proteins. 2025. PMID: 40281335
References
-
- Abbaszadeh F, Fakhri S, Khan H. Targeting apoptosis and autophagy following spinal cord injury: Therapeutic approaches to polyphenols and candidate phytochemicals. Pharmacol Res. 2020;160:105069. - PubMed
-
- Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma. 2006;23:635–659. - PubMed
-
- Benveniste EN. Role of macrophages/microglia in multiple sclerosis and experimental allergic encephalomyelitis. J Mol Med (Berl) 1997;75:165–173. - PubMed
LinkOut - more resources
Full Text Sources

