Combinatorial Atoh1, Gfi1, Pou4f3, and Six1 gene transfer induces hair cell regeneration in the flat epithelium of mature guinea pigs
- PMID: 38103445
- PMCID: PMC11223172
- DOI: 10.1016/j.heares.2023.108916
Combinatorial Atoh1, Gfi1, Pou4f3, and Six1 gene transfer induces hair cell regeneration in the flat epithelium of mature guinea pigs
Abstract
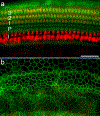
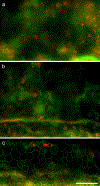
Flat epithelium (FE) is a condition characterized by the loss of both hair cells (HCs) and supporting cells and the transformation of the organ of Corti into a simple flat or cuboidal epithelium, which can occur after severe cochlear insults. The transcription factors Gfi1, Atoh1, Pou4f3, and Six1 (GAPS) play key roles in HC differentiation and survival in normal ears. Previous work using a single transcription factor, Atoh1, to induce HC regeneration in mature ears in vivo usually produced very few cells and failed to produce HCs in severely damaged organs of Corti, especially those with FE. Studies in vitro suggested combinations of transcription factors may be more effective than any single factor, thus the current study aims to examine the effect of co-overexpressing GAPS genes in deafened mature guinea pig cochleae with FE. Deafening was achieved through the infusion of neomycin into the perilymph, leading to the formation of FE and substantial degeneration of nerve fibers. Seven days post neomycin treatment, adenovirus vectors carrying GAPS were injected into the scala media and successfully expressed in the FE. One or two months following GAPS inoculation, cells expressing Myosin VIIa were observed in regions under the FE (located at the scala tympani side of the basilar membrane), rather than within the FE. The number of cells, which we define as induced HCs (iHCs), was not significantly different between one and two months, but the larger N at two months made it more apparent that there were significantly more iHCs in GAPS treated animals than in controls. Additionally, qualitative observations indicated that ears with GAPS gene expression in the FE had more nerve fibers than FE without the treatment. In summary, our results showed that co-overexpression of GAPS enhances the potential for HC regeneration in a severe lesion model of FE.
Keywords: Adenovirus; Cochlea; Deafness; Flat epithelium; Gene transfer; Hair cell regeneration.
Copyright © 2023 Elsevier B.V. All rights reserved.
Figures


Similar articles
-
Response of the flat cochlear epithelium to forced expression of Atoh1.Hear Res. 2008 Jun;240(1-2):52-6. doi: 10.1016/j.heares.2008.02.007. Epub 2008 Mar 7. Hear Res. 2008. PMID: 18430530 Free PMC article.
-
Combinatorial Atoh1 and Gfi1 induction enhances hair cell regeneration in the adult cochlea.Sci Rep. 2020 Dec 8;10(1):21397. doi: 10.1038/s41598-020-78167-8. Sci Rep. 2020. PMID: 33293609 Free PMC article.
-
Generation of mature and functional hair cells by co-expression of Gfi1, Pou4f3, and Atoh1 in the postnatal mouse cochlea.Cell Rep. 2021 Apr 20;35(3):109016. doi: 10.1016/j.celrep.2021.109016. Cell Rep. 2021. PMID: 33882317
-
Atoh1 and other related key regulators in the development of auditory sensory epithelium in the mammalian inner ear: function and interplay.Dev Biol. 2019 Feb 15;446(2):133-141. doi: 10.1016/j.ydbio.2018.12.025. Epub 2018 Dec 31. Dev Biol. 2019. PMID: 30605626 Review.
-
Beyond generalized hair cells: molecular cues for hair cell types.Hear Res. 2013 Mar;297:30-41. doi: 10.1016/j.heares.2012.11.008. Epub 2012 Nov 27. Hear Res. 2013. PMID: 23201032 Free PMC article. Review.
Cited by
-
AAV-mediated Gene Cocktails Enhance Supporting Cell Reprogramming and Hair Cell Regeneration.Adv Sci (Weinh). 2024 Aug;11(29):e2304551. doi: 10.1002/advs.202304551. Epub 2024 May 29. Adv Sci (Weinh). 2024. PMID: 38810137 Free PMC article.
-
Hearing restoration through hair cell regeneration: A review of recent advancements and current limitations.Hear Res. 2025 Jun;461:109256. doi: 10.1016/j.heares.2025.109256. Epub 2025 Mar 22. Hear Res. 2025. PMID: 40157114 Review.
References
-
- Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, Bellen HJ, Lysakowski A, and Zoghbi HY (1999). Math1: an essential gene for the generation of inner ear hair cells. Science 284, 1837–1841. - PubMed
-
- Chen Y, Gu Y, Li Y, Li GL, Chai R, Li W, and Li H (2021). Generation of mature and functional hair cells by co-expression of Gfi1, Pou4f3, and Atoh1 in the postnatal mouse cochlea. Cell Rep 35, 109016. - PubMed
-
- Corwin JT, and Cotanche DA (1988). Regeneration of sensory hair cells after acoustic trauma. Science 240, 1772–1774. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

