Targeting neuraminidase: the next frontier for broadly protective influenza vaccines
- PMID: 38103991
- PMCID: PMC10841738
- DOI: 10.1016/j.it.2023.11.001
Targeting neuraminidase: the next frontier for broadly protective influenza vaccines
Abstract
Current seasonal influenza vaccines, which mainly target hemagglutinin (HA), require annual updates due to the continuous antigenic drift of the influenza virus. Developing an influenza vaccine with increased breadth of protection will have significant public health benefits. The recent discovery of broadly protective antibodies to neuraminidase (NA) has provided important insights into developing a universal influenza vaccine, either by improving seasonal influenza vaccines or designing novel immunogens. However, further in-depth molecular characterizations of NA antibody responses are warranted to fully leverage broadly protective NA antibodies for influenza vaccine designs. Overall, we posit that focusing on NA for influenza vaccine development is synergistic with existing efforts targeting HA, and may represent a cost-effective approach to generating a broadly protective influenza vaccine.
Keywords: antibody; immunogen design; influenza virus; neuraminidase; vaccine.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests N.C.W. is a consultant for HeliXon. The laboratory of A.H.E. received funding under sponsored research agreements from Moderna, Emergent BioSolutions, and AbbVie. A.H.E. has received consulting and speaking fees from InBios International, Inc., Fimbrion Therapeutics, RGAX, Mubadala Investment Company, Moderna, Pfizer, GSK, Danaher, Third Rock Ventures, Goldman Sachs, and Morgan Stanley and is the founder of ImmuneBio Consulting.
Figures
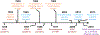
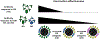
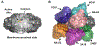
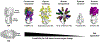
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

