Patient Versus Physician Perspective in the Management of Chronic Myeloid Leukemia During Treatment with Tyrosine Kinase Inhibitors
- PMID: 38104036
- PMCID: PMC10881939
- DOI: 10.1007/s40487-023-00255-2
Patient Versus Physician Perspective in the Management of Chronic Myeloid Leukemia During Treatment with Tyrosine Kinase Inhibitors
Abstract
Introduction: Chronic myeloid leukemia (CML) is a chronic disease with treatment-free remission (TFR) increasingly regarded as a feasible goal of treatment. However, various factors may influence adherence to international guidelines for CML management. This study aimed to compare the reporting of care between patients with CML and their treating doctors.
Methods: Parallel patient and physician online surveys were conducted between September 22, 2021, and March 15, 2022, which focused on the perceptions of 1882 adult patients with CML and 305 physicians regarding tyrosine kinase inhibitor (TKI) treatment options, monitoring and toxicities, TFR, and challenges faced.
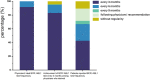
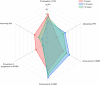
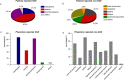
Results: Among the enrolled patients, 69.9% received first-line imatinib treatment, 18.6% received nilotinib, and 4.7% received dasatinib. Among the patients treated with imatinib, 36.7% switched to other TKIs due to imatinib resistance/intolerance (71.1%), exploration of more potent TKIs to achieve TFR (8.9%), and treating physicians' recommendation (14.0%), with a median duration of initial treatment of 14 months [interquartile range (IQR) 6-36]. Most (91.8%) physicians agreed that the breakpoint cluster region-Abelson 1 (BCR::ABL1) transcript level should be assessed every 3 months, but only 42.7% of individuals committed to 3-monthly testing and only 17.8% strictly followed their treating physicians' recommendation. Half of the patients aimed for TFR; however, just 45.2% of physicians considered TFR as one of the top three goals for their patients. The major concern in obtaining TFR was patients' adherence. Fatigue was often distressing for patients with TKIs, while physicians were more concerned about platelet and neutrophil counts. A total of 12% and 20.8% of patients reported moderate/severe anxiety and depression, respectively, while only 53.7% of physicians had concerns about their patients' mental health. During the coronavirus disease 2019 (COVID-19) pandemic, 69.2% of patients reported a reduction in their income. Among these patients, 61.8% maintained their current treatment, while 7.3% switched to cheaper alternatives or discontinued treatment, with over 80% of these patients belonging to the low-income group.
Conclusions: Overcoming challenges in patient-physician communication and treatment access is key to improving disease management and quality of life, especially for patients with low income.
Trial registration: ClinicalTrials.gov identifier NCT05092048.
Keywords: Chronic myeloid leukemia; Discrepancies; Patient; Physician; Quality of life.
© 2023. The Author(s).
Conflict of interest statement
Hong Chen, Yan Wen, Yun Zeng, Lie Lin, Bihong Sun, Hongqian Zhu, Huiqing He, Xiaotao Wang, Waiyi Zou, Caifeng Zheng, Liling Zheng, Jinxiong Huang, Liping Pang, Jixian Huang, Yuming Zhang, Haiqing Lin, Zelin Liu, Wanshou Zhu, Qiang Wang, Xuan Zhou, Xiaoli Liu, Hong Qu, Zhenfang Liu, Xin Du and Na Xu have nothing to disclose.
Figures
References
Associated data
Grants and funding
- 2020A1515010409/Natural Science Foundation of Guangdong Province
- 201904010488/Guangzhou Municipal Science and Technology Bureau
- 82160039/National Natural Science Foundation of China
- 81700162/National Natural Science Foundation of China
- 2020LCZXKF-XY05/the Open project of Yunnan Blood Disease Clinical Medical Center
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

