Longitudinal microbial and molecular dynamics in the cystic fibrosis lung after Elexacaftor-Tezacaftor-Ivacaftor therapy
- PMID: 38104128
- PMCID: PMC10725582
- DOI: 10.1186/s12931-023-02630-z
Longitudinal microbial and molecular dynamics in the cystic fibrosis lung after Elexacaftor-Tezacaftor-Ivacaftor therapy
Abstract
Background: Cystic fibrosis (CF) is a genetic disorder causing poor mucociliary clearance in the airways and subsequent respiratory infection. The recently approved triple therapy Elexacaftor-Tezacaftor-Ivacaftor (ETI) has significantly improved lung function and decreased airway infection in persons with CF (pwCF). This improvement has been shown to occur rapidly, within the first few weeks of treatment. The effects of longer term ETI therapy on lung infection dynamics, however, remain mostly unknown.
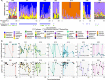
Results: Here, we applied 16S rRNA gene amplicon sequencing, untargeted metabolomics, and neutral models to high-resolution, longitudinally collected sputum samples from pwCF on ETI therapy (162 samples, 7 patients) and compared to similarly collected data set from pwCF not taking ETI (630 samples, 9 patients). Because ETI reduces sputum production, samples were collected in freezers provided in the subject's homes at least 3 months after first taking ETI, with those on ETI collecting a sample approximately weekly. The lung function (%ppFEV1) of those in our longitudinal cohort significantly improved after ETI (6.91, SD = 7.74), indicating our study cohort was responsive to ETI. The daily variation of alpha- and beta-diversity of both the microbiome and metabolome was higher for those on ETI, reflecting a more dynamic microbial community and chemical environment during treatment. Four of the seven subjects on ETI were persistently infected with Pseudomonas or Burkholderia in their sputum throughout the sampling period while the total bacterial load significantly decreased with time (R = - 0.42, p = 0.01) in only one subject. The microbiome and metabolome dynamics on ETI were personalized, where some subjects had a progressive change with time on therapy, whereas others had no association with time on treatment. To further classify the augmented variance of the CF microbiome under therapy, we fit the microbiome data to a Hubbell neutral dynamics model in a patient-stratified manner and found that the subjects on ETI had better fit to a neutral model.
Conclusion: This study shows that the longitudinal microbiology and chemistry in airway secretions from subjects on ETI has become more dynamic and neutral and that after the initial improvement in lung function, many are still persistently infected with CF pathogens.
Keywords: Cystic fibrosis; Elexacaftor–Tezacaftor–Ivacaftor; Lung pathogens; Metabolome; Microbiome; Neutral models; Pseudomonas aeruginosa.
© 2023. The Author(s).
Conflict of interest statement
All authors in the presented study have no conflicts of interest to disclose.
Figures




Update of
-
Longitudinal Microbial and Molecular Dynamics in the Cystic Fibrosis Lung after Elexacaftor-Tezacaftor-Ivacaftor therapy.Res Sq [Preprint]. 2023 Sep 25:rs.3.rs-3356170. doi: 10.21203/rs.3.rs-3356170/v1. Res Sq. 2023. Update in: Respir Res. 2023 Dec 16;24(1):317. doi: 10.1186/s12931-023-02630-z. PMID: 37841851 Free PMC article. Updated. Preprint.
References
-
- Graeber SY, Vitzthum C, Pallenberg ST, Naehrlich L, Stahl M, Rohrbach A, et al. Effects of Elexacaftor/Tezacaftor/Ivacaftor therapy on CFTR function in patients with cystic fibrosis and one or two F508del alleles. Am J Respir Crit Care Med. 2022;205(5):540–549. doi: 10.1164/rccm.202110-2249OC. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

