Mass spectrometry in cerebrospinal fluid uncovers association of glycolysis biomarkers with Alzheimer's disease in a large clinical sample
- PMID: 38104170
- PMCID: PMC10725469
- DOI: 10.1038/s41598-023-49440-3
Mass spectrometry in cerebrospinal fluid uncovers association of glycolysis biomarkers with Alzheimer's disease in a large clinical sample
Abstract
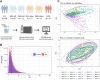
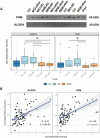
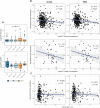
Alzheimer's disease (AD) is a complex and heterogeneous neurodegenerative disorder with contributions from multiple pathophysiological pathways. One of the long-recognized and important features of AD is disrupted cerebral glucose metabolism, but the underlying molecular basis remains unclear. In this study, unbiased mass spectrometry was used to survey CSF from a large clinical cohort, comparing patients who are either cognitively unimpaired (CU; n = 68), suffering from mild-cognitive impairment or dementia from AD (MCI-AD, n = 95; DEM-AD, n = 72), or other causes (MCI-other, n = 77; DEM-other, n = 23), or Normal Pressure Hydrocephalus (NPH, n = 57). The results revealed changes related to altered glucose metabolism. In particular, two glycolytic enzymes, pyruvate kinase (PKM) and aldolase A (ALDOA), were found to be upregulated in CSF from patients with AD compared to those with other neurological conditions. Increases in full-length PKM and ALDOA levels in CSF were confirmed with immunoblotting. Levels of these enzymes furthermore correlated negatively with CSF glucose in matching CSF samples. PKM levels were also found to be increased in AD in publicly available brain-tissue data. These results indicate that ALDOA and PKM may act as technically-robust potential biomarkers of glucose metabolism dysregulation in AD.
© 2023. The Author(s).
Conflict of interest statement
S. Arnold has received honoraria and/or travel expenses for lectures from Abbvie, Eisai, and Biogen and has served on scientific advisory boards of Corte, has received consulting fees from Athira, Cassava, Cognito Therapeutics, EIP Pharma and Orthogonal Neuroscience, and has received research grant support from NIH, Alzheimer’s Association, Alzheimer’s Drug Discovery Foundation, Abbvie, Amylyx, EIP Pharma, Merck, Janssen/Johnson & Johnson, Novartis, and vTv. S.N. Leslie is a current employee of Janssen Pharmaceuticals. B. Carlyle has received grant funding from Ono Pharmaceutical. Other authors report no conflicts of interest.
Figures


Update of
-
Mass Spectrometry in Cerebrospinal Fluid Uncovers Association of Glycolysis Biomarkers with Alzheimer's Disease in a Large Clinical Sample.Res Sq [Preprint]. 2023 Jun 27:rs.3.rs-3073597. doi: 10.21203/rs.3.rs-3073597/v1. Res Sq. 2023. Update in: Sci Rep. 2023 Dec 16;13(1):22406. doi: 10.1038/s41598-023-49440-3. PMID: 37461556 Free PMC article. Updated. Preprint.
References
-
- Santos CY, Snyder PJ, Wu WC, Zhang M, Echeverria A, Alber J. Pathophysiologic relationship between Alzheimer’s disease, cerebrovascular disease, and cardiovascular risk: A review and synthesis. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2017;7(1):69–87. doi: 10.1016/J.DADM.2017.01.005. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

