Role of Fra-2 in cancer
- PMID: 38104183
- PMCID: PMC10850073
- DOI: 10.1038/s41418-023-01248-4
Role of Fra-2 in cancer
Abstract
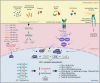
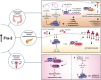
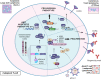
Fos-related antigen-2 (Fra-2) is the most recently discovered member of the Fos family and, by dimerizing with Jun proteins, forms the activator protein 1 (AP-1) transcription factor. By inducing or repressing the transcription of several target genes, Fra-2 is critically involved in the modulation of cell response to a variety of extracellular stimuli, stressors and intracellular changes. In physiological conditions, Fra-2 has been found to be ubiquitously expressed in human cells, regulating differentiation and homeostasis of bone, muscle, nervous, lymphoid and other tissues. While other AP-1 members, like Jun and Fos, are well characterized, studies of Fra-2 functions in cancer are still at an early stage. Due to the lack of a trans-activating domain, which is present in other Fos proteins, it has been suggested that Fra-2 might inhibit cell transformation, eventually exerting an anti-tumor effect. In human malignancies, however, Fra-2 activity is enhanced (or induced) by dysregulation of microRNAs, oncogenes and extracellular signaling, suggesting a multifaceted role. Therefore, Fra-2 can promote or prevent transformation, proliferation, migration, epithelial-mesenchymal transition, drug resistance and metastasis formation in a tumor- and context-dependent manner. Intriguingly, recent data reports that Fra-2 is also expressed in cancer associated cells, contributing to the intricate crosstalk between neoplastic and non-neoplastic cells, that leads to the evolution and remodeling of the tumor microenvironment. In this review we summarize three decades of research on Fra-2, focusing on its oncogenic and anti-oncogenic effects in tumor progression and dissemination.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Shaulian E, Karin M. AP-1 as a regulator of cell life and death. Nat Cell Biol. 2002;4:E131–136. - PubMed
-
- Sun L, Guo Z, Sun J, Li J, Dong Z, Zhang Y, et al. MiR-133a acts as an anti-oncogene in Hepatocellular carcinoma by inhibiting FOSL2 through TGF-β/Smad3 signaling pathway. Biomed Pharmacother. 2018;107:168–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

