Standard-of-care systemic therapy with or without stereotactic body radiotherapy in patients with oligoprogressive breast cancer or non-small-cell lung cancer (Consolidative Use of Radiotherapy to Block [CURB] oligoprogression): an open-label, randomised, controlled, phase 2 study
- PMID: 38104577
- PMCID: PMC10880046
- DOI: 10.1016/S0140-6736(23)01857-3
Standard-of-care systemic therapy with or without stereotactic body radiotherapy in patients with oligoprogressive breast cancer or non-small-cell lung cancer (Consolidative Use of Radiotherapy to Block [CURB] oligoprogression): an open-label, randomised, controlled, phase 2 study
Abstract
Background: Most patients with metastatic cancer eventually develop resistance to systemic therapy, with some having limited disease progression (ie, oligoprogression). We aimed to assess whether stereotactic body radiotherapy (SBRT) targeting oligoprogressive sites could improve patient outcomes.
Methods: We did a phase 2, open-label, randomised controlled trial of SBRT in patients with oligoprogressive metastatic breast cancer or non-small-cell lung cancer (NSCLC) after having received at least first-line systemic therapy, with oligoprogression defined as five or less progressive lesions on PET-CT or CT. Patients aged 18 years or older were enrolled from a tertiary cancer centre in New York, NY, USA, and six affiliated regional centres in the states of New York and New Jersey, with a 1:1 randomisation between standard of care (standard-of-care group) and SBRT plus standard of care (SBRT group). Randomisation was done with a computer-based algorithm with stratification by number of progressive sites of metastasis, receptor or driver genetic alteration status, primary site, and type of systemic therapy previously received. Patients and investigators were not masked to treatment allocation. The primary endpoint was progression-free survival, measured up to 12 months. We did a prespecified subgroup analysis of the primary endpoint by disease site. All analyses were done in the intention-to-treat population. The study is registered with ClinicalTrials.gov, NCT03808662, and is complete.
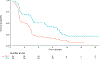
Findings: From Jan 1, 2019, to July 31, 2021, 106 patients were randomly assigned to standard of care (n=51; 23 patients with breast cancer and 28 patients with NSCLC) or SBRT plus standard of care (n=55; 24 patients with breast cancer and 31 patients with NSCLC). 16 (34%) of 47 patients with breast cancer had triple-negative disease, and 51 (86%) of 59 patients with NSCLC had no actionable driver mutation. The study was closed to accrual before reaching the targeted sample size, after the primary efficacy endpoint was met during a preplanned interim analysis. The median follow-up was 11·6 months for patients in the standard-of-care group and 12·1 months for patients in the SBRT group. The median progression-free survival was 3·2 months (95% CI 2·0-4·5) for patients in the standard-of-care group versus 7·2 months (4·5-10·0) for patients in the SBRT group (hazard ratio [HR] 0·53, 95% CI 0·35-0·81; p=0·0035). The median progression-free survival was higher for patients with NSCLC in the SBRT group than for those with NSCLC in the standard-of-care group (10·0 months [7·2-not reached] vs 2·2 months [95% CI 2·0-4·5]; HR 0·41, 95% CI 0·22-0·75; p=0·0039), but no difference was found for patients with breast cancer (4·4 months [2·5-8·7] vs 4·2 months [1·8-5·5]; 0·78, 0·43-1·43; p=0·43). Grade 2 or worse adverse events occurred in 21 (41%) patients in the standard-of-care group and 34 (62%) patients in the SBRT group. Nine (16%) patients in the SBRT group had grade 2 or worse toxicities related to SBRT, including gastrointestinal reflux disease, pain exacerbation, radiation pneumonitis, brachial plexopathy, and low blood counts.
Interpretation: The trial showed that progression-free survival was increased in the SBRT plus standard-of-care group compared with standard of care only. Oligoprogression in patients with metastatic NSCLC could be effectively treated with SBRT plus standard of care, leading to more than a four-times increase in progression-free survival compared with standard of care only. By contrast, no benefit was observed in patients with oligoprogressive breast cancer. Further studies to validate these findings and understand the differential benefits are warranted.
Funding: National Cancer Institute.
Copyright © 2024 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests CJT is on the advisory board of Nanobiotix and Varian Medical, and received consultation fees and honorarium support from Varian Medical. AR received grants from Varian Medical, AstraZeneca, Merck, Boehringer Ingelheim, Pfizer, and the National Cancer Institute at the National Institutes of Health. DRG is a researcher with AstraZeneca, Bristol Myers Squibb, and Merck; is on the advisory board of Grail, Olympus, Johnson & Johnson, Varian Medical, and Medtronic; and served as a speaker for MedLearning Group. NYL is on the advisory board of Merck, Merck EMD, Nanobiotix, and Galera Therapeutics; and received consulting fees from Shanghai JoAnn Medical Technology, Yingming Consulting, and Varian Medical. NR received research support from Invitae. All other authors declare no competing interests.
Figures





Comment in
-
SBRT for oligoprogressive disease: using the evidence to maximise the benefits.Lancet. 2024 Jan 13;403(10422):122-124. doi: 10.1016/S0140-6736(23)02351-6. Epub 2023 Dec 14. Lancet. 2024. PMID: 38104574 No abstract available.
-
The heterogeneity in oligoprogression and stereotactic body radiation therapy.Transl Cancer Res. 2025 Jan 31;14(1):1-6. doi: 10.21037/tcr-24-1737. Epub 2025 Jan 21. Transl Cancer Res. 2025. PMID: 39974422 Free PMC article. No abstract available.
References
-
- Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol 1995; 13: 8–10. - PubMed
-
- Pastorino U, Buyse M, Friedel G, et al. Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg 1997; 113: 37–49. - PubMed
-
- Ost P, Jereczek-Fossa BA, As NV, et al. Progression-free Survival Following Stereotactic Body Radiotherapy for Oligometastatic Prostate Cancer Treatment-naive Recurrence: A Multi-institutional Analysis. Eur Urol 2016; 69: 9–12. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

