MiR-146b-5p enriched bioinspired exosomes derived from fucoidan-directed induction mesenchymal stem cells protect chondrocytes in osteoarthritis by targeting TRAF6
- PMID: 38105181
- PMCID: PMC10726686
- DOI: 10.1186/s12951-023-02264-9
MiR-146b-5p enriched bioinspired exosomes derived from fucoidan-directed induction mesenchymal stem cells protect chondrocytes in osteoarthritis by targeting TRAF6
Abstract
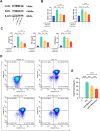
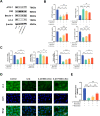
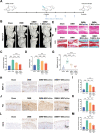
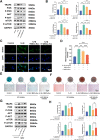
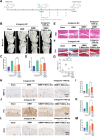
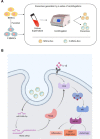
Osteoarthritis (OA) is a common degenerative joint disease characterized by progressive cartilage degradation and inflammation. In recent years, mesenchymal stem cells (MSCs) derived exosomes (MSCs-Exo) have attracted widespread attention for their potential role in modulating OA pathology. However, the unpredictable therapeutic effects of exosomes have been a significant barrier to their extensive clinical application. In this study, we investigated whether fucoidan-pretreated MSC-derived exosomes (F-MSCs-Exo) could better protect chondrocytes in osteoarthritic joints and elucidate its underlying mechanisms. In order to evaluate the role of F-MSCs-Exo in osteoarthritis, both in vitro and in vivo studies were conducted. MiRNA sequencing was employed to analyze MSCs-Exo and F-MSCs-Exo, enabling the identification of differentially expressed genes and the exploration of the underlying mechanisms behind the protective effects of F-MSCs-Exo in osteoarthritis. Compared to MSCs-Exo, F-MSCs-Exo demonstrated superior effectiveness in inhibiting inflammatory responses and extracellular matrix degradation in rat chondrocytes. Moreover, F-MSCs-Exo exhibited enhanced activation of autophagy in chondrocytes. MiRNA sequencing of both MSCs-Exo and F-MSCs-Exo revealed that miR-146b-5p emerged as a promising candidate mediator for the chondroprotective function of F-MSCs-Exo, with TRAF6 identified as its downstream target. In conclusion, our research results demonstrate that miR-146b-5p encapsulated in F-MSCs-Exo effectively inhibits TRAF6 activation, thereby suppressing inflammatory responses and extracellular matrix degradation, while promoting chondrocyte autophagy for the protection of osteoarthritic cartilage cells. Consequently, the development of a therapeutic approach combining fucoidan with MSC-derived exosomes provides a promising strategy for the clinical treatment of osteoarthritis.
Keywords: Exosomes; Fucoidan; MSCs; Osteoarthritis; TRAF6; miR-146b-5p.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

