Depletion of muscularis macrophages ameliorates inflammation-driven dysmotility in murine colitis model
- PMID: 38105266
- PMCID: PMC10725888
- DOI: 10.1038/s41598-023-50059-7
Depletion of muscularis macrophages ameliorates inflammation-driven dysmotility in murine colitis model
Abstract
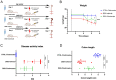
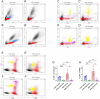
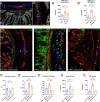
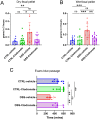
Previously, the presence of a blood-myenteric plexus barrier and its disruption was reported in experimentally induced colitis via a macrophage-dependent process. The aim of this study is to reveal how myenteric barrier disruption and subsequent neuronal injury affects gut motility in vivo in a murine colitis model. We induced colitis with dextran sulfate sodium (DSS), with the co-administration of liposome-encapsulated clodronate (L-clodronate) to simultaneously deplete blood monocytes contributing to macrophage infiltration in the inflamed muscularis of experimental mice. DSS-treated animals receiving concurrent L-clodronate injection showed significantly decreased blood monocyte numbers and colon muscularis macrophage (MM) density compared to DSS-treated control (DSS-vehicle). DSS-clodronate-treated mice exhibited significantly slower whole gut transit time than DSS-vehicle-treated animals and comparable to that of controls. Experiments with oral gavage-fed Evans-blue dye showed similar whole gut transit times in DSS-clodronate-treated mice as in control animals. Furthermore, qPCR-analysis and immunofluorescence on colon muscularis samples revealed that factors associated with neuroinflammation and neurodegeneration, including Bax1, Hdac4, IL-18, Casp8 and Hif1a are overexpressed after DSS-treatment, but not in the case of concurrent L-clodronate administration. Our findings highlight that MM-infiltration in the muscularis layer is responsible for colitis-associated dysmotility and enteric neuronal dysfunction along with the release of mediators associated with neurodegeneration in a murine experimental model.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Mogor F, Kovács T, Lohinai Z, Dora D. The enteric nervous system and the microenvironment of the gut: The translational aspects of the microbiome-gut-brain axis. Appl. Sci. 2021;11(24):12000. doi: 10.3390/app1124120. - DOI
-
- Muller PA, Koscsó B, Rajani GM, Stevanovic K, Berres ML, Hashimoto D, Mortha A, Leboeuf M, Li XM, Mucida D, Stanley ER, Dahan S, Margolis KG, Gershon MD, Merad M, Bogunovic M. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell. 2014;158(2):300–313. doi: 10.1016/j.cell.2014.04.050. - DOI - PMC - PubMed
-
- De Schepper S, Verheijden S, Aguilera-Lizarraga J, Viola MF, Boesmans W, Stakenborg N, Voytyuk I, Schmidt I, Boeckx B, Dierckx de Casterlé I, Baekelandt V, Gonzalez Dominguez E, Mack M, Depoortere I, De Strooper B, Sprangers B, Himmelreich U, Soenen S, Guilliams M, Vanden Berghe P, Jones E, Lambrechts D, Boeckxstaens G. Self-Maintaining gut macrophages are essential for intestinal homeostasis. Cell. 2018;175(2):400–41513. doi: 10.1016/j.cell.2018.07.048. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- RRF-2.3.1-21-2022-00011/National Laboratory for Translational Neuroscience
- #142287/National Research, Development and Innovation Office
- #138664/National Research, Development and Innovation Office
- #134751/National Research, Development and Innovation Office
- 739593/European Union's Horizon 2020 Research and Innovation Programme
LinkOut - more resources
Full Text Sources
Miscellaneous

