Epeleuton, a novel synthetic ω-3 fatty acid, reduces hypoxia/ reperfusion stress in a mouse model of sickle cell disease
- PMID: 38105727
- PMCID: PMC11141675
- DOI: 10.3324/haematol.2023.284028
Epeleuton, a novel synthetic ω-3 fatty acid, reduces hypoxia/ reperfusion stress in a mouse model of sickle cell disease
Abstract
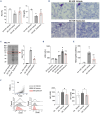
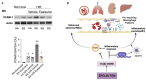
Inflammatory vasculopathy is critical in sickle cell disease (SCD)-associated organ damage. An imbalance between pro-inflammatory and pro-resolving mechanisms in response to different triggers such as hypoxia/reoxygenation or infections has been proposed to contribute to the progression of SCD. Administration of specialized pro-resolving lipid mediators may provide an effective therapeutic strategy to target inflammatory vasculopathy and to modulate inflammatory response. Epeleuton (15 hydroxy eicosapentaenoic acid ethyl ester) is a novel, orally administered, second-generation ω-3 fatty acid with a favorable clinical safety profile. In this study we show that epeleuton re-programs the lipidomic pattern of target organs for SCD towards a pro-resolving pattern. This protects against systemic and local inflammatory responses and improves red cell features, resulting in reduced hemolysis and sickling compared with that in vehicle-treated SCD mice. In addition, epeleuton prevents hypoxia/reoxygenation-induced activation of nuclear factor-κB with downregulation of the NLRP3 inflammasome in lung, kidney, and liver. This was associated with downregulation of markers of vascular activation in epeleuton-treated SCD mice when compared to vehicle-treated animals. Collectively our data support the potential therapeutic utility of epeleuton and provide the rationale for the design of clinical trials to evaluate the efficacy of epeleuton in patients with SCD.
Figures





References
-
- Bunn HF. Pathogenesis and treatment of sickle cell disease. N Engl J Med. 1997;337(11):762-769. - PubMed
-
- Hebbel RP. The systems biology-based argument for taking a bold step in chemoprophylaxis of sickle vasculopathy. Am J Hematol. 2009;84(9):543-545. - PubMed
-
- Serhan CN, Jain A, Marleau S, et al. . Reduced inflammation and tissue damage in transgenic rabbits overexpressing 15-lipoxygenase and endogenous anti-inflammatory lipid mediators. J Immunol. 2003;171(12):6856-6865. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

