Immunology of amyotrophic lateral sclerosis - role of the innate and adaptive immunity
- PMID: 38105925
- PMCID: PMC10723830
- DOI: 10.3389/fnins.2023.1277399
Immunology of amyotrophic lateral sclerosis - role of the innate and adaptive immunity
Abstract
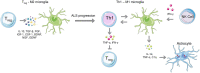
This review aims to summarize the latest evidence about the role of innate and adaptive immunity in Amyotrophic Lateral Sclerosis (ALS). ALS is a devastating neurodegenerative disease affecting upper and lower motor neurons, which involves essential cells of the immune system that play a basic role in innate or adaptive immunity, that can be neurotoxic or neuroprotective for neurons. However, distinguishing between the sole neurotoxic or neuroprotective function of certain cells such as astrocytes can be challenging due to intricate nature of these cells, the complexity of the microenvironment and the contextual factors. In this review, in regard to innate immunity we focus on the involvement of monocytes/macrophages, microglia, the complement, NK cells, neutrophils, mast cells, and astrocytes, while regarding adaptive immunity, in addition to humoral immunity the most important features and roles of T and B cells are highlighted, specifically different subsets of CD4+ as well as CD8+ T cells. The role of autoantibodies and cytokines is also discussed in distinct sections of this review.
Keywords: adaptive immune system; amyotrophic lateral sclerosis; innate immune system; neurodegeneration; neuroimmunology and neuropathy.
Copyright © 2023 Mimic, Aru, Pehlivanoğlu, Sleiman, Andjus and Yanıkkaya Demirel.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

