This is a preprint.
Tumor-selective effects of active RAS inhibition in pancreatic ductal adenocarcinoma
- PMID: 38105998
- PMCID: PMC10723304
- DOI: 10.1101/2023.12.03.569791
Tumor-selective effects of active RAS inhibition in pancreatic ductal adenocarcinoma
Abstract
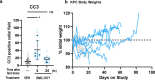
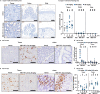
Broad-spectrum RAS inhibition holds the potential to benefit roughly a quarter of human cancer patients whose tumors are driven by RAS mutations. However, the impact of inhibiting RAS functions in normal tissues is not known. RMC-7977 is a highly selective inhibitor of the active (GTP-bound) forms of KRAS, HRAS, and NRAS, with affinity for both mutant and wild type (WT) variants. As >90% of human pancreatic ductal adenocarcinoma (PDAC) cases are driven by activating mutations in KRAS, we assessed the therapeutic potential of RMC-7977 in a comprehensive range of PDAC models, including human and murine cell lines, human patient-derived organoids, human PDAC explants, subcutaneous and orthotopic cell-line or patient derived xenografts, syngeneic allografts, and genetically engineered mouse models. We observed broad and pronounced anti-tumor activity across these models following direct RAS inhibition at doses and concentrations that were well-tolerated in vivo. Pharmacological analyses revealed divergent responses to RMC-7977 in tumor versus normal tissues. Treated tumors exhibited waves of apoptosis along with sustained proliferative arrest whereas normal tissues underwent only transient decreases in proliferation, with no evidence of apoptosis. Together, these data establish a strong preclinical rationale for the use of broad-spectrum RAS inhibition in the setting of PDAC.
Conflict of interest statement
Competing interests J.J., Y.W., B.L., M.M., S.C., L.J., X.W., Y.C.Y., C.H., H.C., Y.G., R.Z., E.Q., Z.W., J.A.M.S., M.H., D.W., and M.S. are employees and stockholders of Revolution Medicines. R.H.V., B.Z.S., A.J.A., C.J.D., and K.P.O. received research funding from Revolution Medicines. A.J.A. consults for Anji Pharmaceuticals, Affini-T Therapeutics, Arrakis Therapeutics, AstraZeneca, Boehringer Ingelheim, Oncorus, Inc., Merck & Co. Inc., Mirati Therapeutics, Nimbus Therapeutics, Plexium, Revolution Medicines, Reactive Biosciences, Riva Therapeutics, Servier Pharmaceuticals, Syros Pharmaceuticals, T-knife Therapeutics, Third Rock Ventures, and Ventus Therapeutics. A.J.A. holds equity in Riva Therapeutics. A.J.A. has research funding from Bristol Myers Squibb, Deerfield, Inc., Eli Lilly, Mirati Therapeutics, Novartis, Novo Ventures, and Syros Pharmaceuticals. C.J.D. is a consultant/advisory board member for Cullgen, Deciphera Pharmaceuticals, Eli Lilly, Mirati Therapeutics, Reactive Biosciences, Revolution Medicines, Ribometrics, Sanofi, and SHY Therapeutics. C.J.D. has received research funding support from Deciphera Pharmaceuticals, Mirati Therapeutics, Reactive Biosciences, and SpringWorks Therapeutics. R.H.V. has received consulting fees from BMS, is an inventor on patents relating to cancer cellular immunotherapy, cancer vaccines, and KRAS immune epitopes, and receives royalties from Children’s Hospital Boston for a licensed research-only monoclonal antibody.
Figures







References
Publication types
Grants and funding
- U01 CA272610/CA/NCI NIH HHS/United States
- R01 CA042978/CA/NCI NIH HHS/United States
- R01 CA266558/CA/NCI NIH HHS/United States
- T32 CA009156/CA/NCI NIH HHS/United States
- T32 CA009140/CA/NCI NIH HHS/United States
- U01 CA274312/CA/NCI NIH HHS/United States
- U01 CA199235/CA/NCI NIH HHS/United States
- R01 CA276268/CA/NCI NIH HHS/United States
- T32 CA071341/CA/NCI NIH HHS/United States
- R01 CA229803/CA/NCI NIH HHS/United States
- P30 CA013696/CA/NCI NIH HHS/United States
- P50 CA196510/CA/NCI NIH HHS/United States
- U54 CA274506/CA/NCI NIH HHS/United States
- R01 CA215607/CA/NCI NIH HHS/United States
- P01 CA203657/CA/NCI NIH HHS/United States
- R35 CA232113/CA/NCI NIH HHS/United States
- S10 OD032433/OD/NIH HHS/United States
- P30 DK132710/DK/NIDDK NIH HHS/United States
- R01 CA175747/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
