This is a preprint.
Eribulin induces micronuclei and enhances the nuclear localization of cGAS in triple-negative breast cancer cells
- PMID: 38106033
- PMCID: PMC10723555
- DOI: 10.21203/rs.3.rs-3672056/v1
Eribulin induces micronuclei and enhances the nuclear localization of cGAS in triple-negative breast cancer cells
Update in
-
Eribulin induces micronuclei and enhances the nuclear localization of cGAS in triple-negative breast cancer cells.Sci Rep. 2024 Jun 19;14(1):14146. doi: 10.1038/s41598-024-64651-y. Sci Rep. 2024. PMID: 38898119 Free PMC article.
Abstract
Eribulin (ERI), clinically utilized for locally advanced or metastatic breast tumors, has shown potential links to the immune system. Notably, the cGAS-STING pathway, a key component of innate immunity, has gained prominence. Yet, limited reports explore ERI's effects on the cGAS-STING pathway. Additionally, the nuclear presence of cGAS remains poorly understood. This study uniquely delves into ERI's impact on both the cytosolic cGAS-STING pathway and nuclear cGAS. ERI enhances nuclear localization of cGAS, resulting in hyper-activation of the cGAS-STING pathway in triple-negative breast cancer cells. Reduction of cGAS heightened both cell proliferation and ERI sensitivity. In clinical data using ERI in a neo-adjuvant setting, patients with low cGAS cases exhibited reduced likelihood of achieving pathological complete response after ERI treatment. These findings illuminate the potential of cGAS and IFNβ as predictive biomarkers for ERI sensitivity, providing valuable insights for personalized breast cancer treatment strategies.
Conflict of interest statement
Competing interests The authors declare no competing interests. This work was supported by JSPS KAKENHI Grant Number JP22K08727 and JP21K08638
Figures

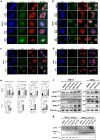


References
-
- Cortes Javier et al. Eribulin monotherapy versus treatment of physician’s choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet. 377, 914–923 (2011) - PubMed
-
- Kazutaka N et al. A Randomized Controlled Phase 2 Study of Neoadjuvant Eribulin Versus Paclitaxel in Women with Operable Breast Cancer: The JONIE-3 Study. Clinical Breast Cancer. 22, e881–e891 (2022) - PubMed
-
- Lengauer C K W Kinzler, B Vogelstein. Genetic instabilities in human cancers. Nature. 396, 643–649 (1998). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

