This is a preprint.
Whole genome sequencing refines stratification and therapy of patients with clear cell renal cell carcinoma
- PMID: 38106039
- PMCID: PMC10723546
- DOI: 10.21203/rs.3.rs-3675752/v1
Whole genome sequencing refines stratification and therapy of patients with clear cell renal cell carcinoma
Update in
-
Whole genome sequencing refines stratification and therapy of patients with clear cell renal cell carcinoma.Nat Commun. 2024 Jul 15;15(1):5935. doi: 10.1038/s41467-024-49692-1. Nat Commun. 2024. PMID: 39009593 Free PMC article.
Abstract
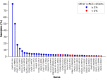
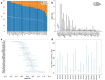
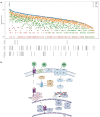
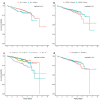
Clear cell renal cell carcinoma (ccRCC) is the most common form of kidney cancer, but a comprehensive description of its genomic landscape is lacking. We report the whole genome sequencing of 778 ccRCC patients enrolled in the 100,000 Genomes Project, providing the most detailed somatic mutational landscape to date. We identify new driver genes, which as well as emphasising the major role of epigenetic regulation in ccRCC highlight additional biological pathways extending opportunities for drug repurposing. Genomic characterisation identified patients with divergent clinical outcome; higher number of structural copy number alterations associated with poorer prognosis, whereas VHL mutations were independently associated with a better prognosis. The twin observations that higher T-cell infiltration is associated with better outcome and that genetically predicted immune evasion is not common supports the rationale for immunotherapy. These findings should inform personalised surveillance and treatment strategies for ccRCC patients.
Conflict of interest statement
DECLARATION OF INTERESTS S.T. has received speaking fees from Roche, AstraZeneca, Novartis and Ipsen. ST has the following patents filed: Indel mutations as a therapeutic target and predictive biomarker PCTGB2018/051892 and PCTGB2018/051893 and Clear Cell Renal Cell Carcinoma Biomarkers P113326GB. None of the other authors have a conflict of interest.
Figures




References
-
- Bukavina L. et al..Epidemiology of Renal Cell Carcinoma: 2022 Update.Eur. Urol. 82,529–542(2022). - PubMed
-
- Post nephrectomy management of localized renal cell carcinoma.From risk stratification to therapeutic evidence in an evolving clinical scenario.Cancer Treat. Rev.115,102528(2023). - PubMed
-
- Choueiri T. K. et al..Adjuvant Pembrolizumab after Nephrectomy in Renal-Cell Carcinoma.N. Engl. J. Med.385,683–694(2021). - PubMed
-
- Pal S. K. et al..Adjuvant atezolizumab versus placebo for patients with renal cell carcinoma at increased risk of recurrence following resection (IMmotion010): a multicentre, randomised, double-blind, phase 3 trial.Lancet 400,1103–1116(2022). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

